The MRI Safety Gap
The MRI Safety Gap
In healthcare, particularly in patient safety, there is a cultural predisposition towards excellence. There’s a fundamental desire to create better, safer environments in support of care. That applies to staff qualifications, policies & procedures, medical technology, and — usually — standards for accreditation.
I say ‘usually’ because there is a glaring hole, more than two decades old, in patient safety accreditation standards: MRI (magnetic resonance imaging).
Approximately 1 in 10 Americans — or roughly 30,000,000 people — had an MRI last year. Most if not all of them went through some type of screening and passed signs with cryptic warnings as they entered locked doors to the MRI suite. The screening and warnings are intended to prevent serious accidents and injuries. Ferromagnetic materials (such as oxygen tanks, wheelchairs, cleaning equipment) must be kept outside the MRI suite lest they become magnet-homing missiles, which have killed patients in the past. Patients with contraindicated implants may experience potentially fatal adverse interactions with the MRI’s magnetic field or RF energies, and facilities must prevent MRI devices, which can cost in excess of $2 million, from accidental damage.
![]()
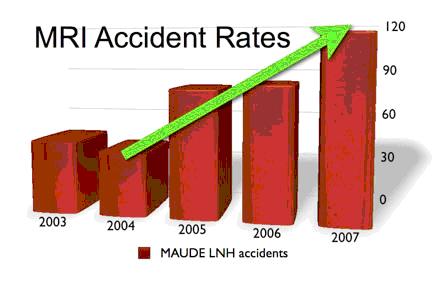
Numbers of MRI accidents (product code ‘LNH’) reported to the FDA’s MAUDE database over the last several years have seen a 185% increase from 2004 to 2007. MAUDE data for 2008 is not complete, but already shows an almost 20% increase over 2007 with only 11 months of data available.
The FDA’s account of MRI accident rates has nearly tripled in the past four yearsi, but there is not one explicit site-specific MRI safety requirement in any accreditation standardii. As of January 1st, however, changes to the Joint Commission Environment of Care (EC) standard present an opportunity for the first substantive move towards MRI safety standardization.
One of the new requirements under the EC standard is for accredited facilities to use, as a minimum, all previously published Sentinel Event Alerts — the Joint Commission’s highest patient safety warning — to assess risk. Should a particular Alert apply to the facilities or practices of the provider, the risk assessment must detail whether the facility complies with current best practices for each identified hazard or patient safety objective enumerated in the Alert.
Last February, the Joint Commission released Sentinel Event Alert #38iii, MRI Accidents and Injuries, which details 10 explicit safety objectives (plus another three in the body of the Alert) for improving safety in the MRI suite. Because the objectives identified in the Alert pertain to the general MRI environment (as opposed to specific MRI devices or particular clinical settings), the Alert applies universally to all Joint Commission accredited providers of MRI services.
So — perhaps unknowingly — the Joint Commission, through their EC standard update, has established a requirement that accredited MRI providers conduct a risk assessment. What does this mean in practice? Absolutely nothing, unless the Joint Commission chooses to enforce the new standard.
The Joint Commission can become a victim of it’s own institutional inertia, simply ignore the fact that the new EC standard applies to MRI safety, and continue the legacy of inaction, or it can honor the new standard and verify that accredited providers have conducted their own evaluations of risk factors.
I encourage the Joint Commission to hold itself to at least a ‘B-‘ standard with regard to the new EC provisions for MRI safety, namely that they verify the presence of an MRI risk assessment in at least 80% of surveyed accredited MRI providers.
Stunning growth in MRI accident reports, Sentinel Event Alert #38, the new EC standards, and a desire to catch-up after 20 years of accrediting hospitals without applying MRI-specific safety standards should be all the motivation needed to spur serious improvements for the benefit of MRI patients, their families, as well as their caregivers.
Tobias Gilk is a member of the American College of Radiology’s (ACR) MR Safety Committee and President and MRI Safety Director for Mednovus, Inc. He has written hundreds of articles on MRI safety (including a few for the Joint Commission) and is a popular speaker on the subject.
ACR Guidance Document for Safe MR Practices: 2007 http://www.acr.org/mr_safety
VA National Center for Patient Safety: MR Hazard Summary
http://www.patientsafety.gov/SafetyTopics/mrihazardsummary.html
“New Tools for MRI Safety: Ferromagnetically Naked”, PSQH http://www.psqh.com/julaug08/mri-safety.html
