Taking a DNA “Time Out” to Ensure Accuracy
July / August 2012
![]()
Taking a DNA “Time Out” to Ensure Accuracy
According to the American Cancer Society, breast cancer is the second most common cancer among women in the United States. In fact, it accounts for nearly one in three cancers diagnosed in U.S. women (2012).
These astounding statistics translate to approximately 1.6 million breast biopsies being performed on an annual basis in the U.S. as part of the diagnostic testing cycle following detection of a suspicious lesion during a mammography screening. Of these 1.6 million biopsies, approximately 20% will result in a positive cancer diagnosis (Silverstein et al., 2005). To help eradicate the cancer and prevent its metastasis to other organs, the proper diagnosis of lesions is necessary to ensure the appropriate course of treatment is employed. Depending on the stage of cancer, treatment may include a full or partial mastectomy, chemotherapy, and radiation therapy.
However, many patients are not aware that after the biopsy occurs, the tissue samples move through a complex process to determine whether cells are cancerous and, if so, what stage of cancer is present. This opens up the possibility of human error at almost every stage of the process; these potential errors are referred to as Specimen Provenance Complications (SPCs).
Given the complex nature of the diagnostic testing cycle, preventing diagnostic mistakes due to SPCs is crucial to facilitating proper treatment and optimal patient outcomes.
The Diagnostic Process and Potential for SPC Errors
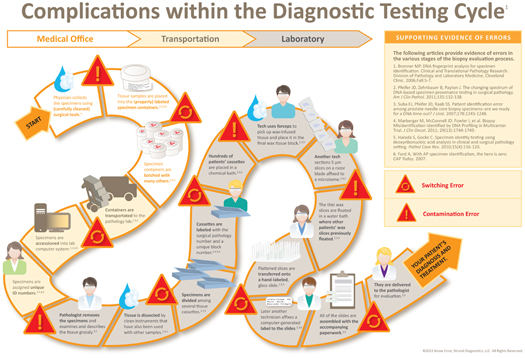
As mentioned above, the diagnostic testing cycle requires as many as 18 steps and several medical professionals working in different locations. At each step in the process, there is potential for human error to occur. Examples of SPCs in the biopsy evaluation process include mislabeling, specimen transposition, and foreign cell contamination (Figure 1). As a result, it is estimated that the findings of approximately 1 in every 100 biopsies performed in the United States result in a non-DNA match—delaying the treatment of the patient with cancer and unnecessarily treating those patients who do not have cancer.
Figure 1: Complications withing the Diagnostic Testing Cycle
A positive cancer diagnosis often takes a large emotional toll on patients and their families. Reporting biopsy results to the wrong individual can have a life-changing impact on multiple stakeholders. Patients who receive a false positive diagnosis or whose cancer is not properly staged may be subject to drastic intervention including lumpectomy, mastectomies, chemotherapy, and radiation on non-diseased tissue. Conversely, patients who receive a false negative diagnosis may be delaying potentially life-saving treatments.
Recently, I was told of the following case by a colleague: a 54-year-old female was diagnosed with invasive ductal carcinoma in her right breast after undergoing an ultrasound-guided breast biopsy at her physician’s office. Less than two months after the diagnosis was rendered, a simple/partial mastectomy was performed to treat the cancer. However, when the extracted breast tissue was examined by a pathologist following the surgery, no cancer cells were detected. Further investigation comparing the biopsy tissue against DNA of all patients biopsied in the physician’s office the same day as the 54-year old patient revealed a specimen transposition error between patient tissue samples. The wrong woman was diagnosed with breast cancer. While it’s unclear how this SPC occurred, the situation resulted in misdiagnosis and unnecessary surgery for one patient and a delay in treatment for the other patient.
Identifying Misidentification and Contamination Errors
Providing the highest quality care and avoiding undesired outcomes is what we as physicians strive to achieve without exception for our patients. At Bridgeport Hospital, we are able to ensure a patient’s diagnosis is truly her own by incorporating DNA Specimen Provenance Assignment (DSPA) testing as a standard part of our breast and prostate biopsy protocol, through our use of the Know Error system. The DSPA testing available in this system reduces the incidence of SPCs so that diagnostic mistakes are minimized and the opportunity for negative outcomes is diminished.
DSPA testing is used to definitively assign specimen identity when making the diagnosis of cancer and other histopathological conditions. At the time of the biopsy procedure, a buccal swab of the patient’s cheek is taken to obtain a DNA reference sample. The swab is then sent to an independent DNA laboratory. The biopsy tissue samples are collected as usual then placed in barcoded specimen containers, matching that of the buccal swab, and sent to the pathology lab for evaluation. If a malignancy is found in a biopsy sample, a DSPA test is completed to compare the DNA profiles of the biopsy tissue against the reference DNA sample. Concurrence of these profiles allows for absolute confirmation of patient identity and is then utilized to outline an appropriate treatment course.
Benefits of a DNA “Time Out”
Adding this DNA confirmation—taking a DNA “Time Out”—completes the diagnostic testing cycle and provides physicians and patients alike with the assurance that the positive biopsy specimen is that of the patient in question. This confirmation ensures that physicians are armed with accurate information to make better informed treatment recommendations for patients. Moreover, harnessing the power of DNA confirmation reduces the likelihood of over-treatment or under-treatment of our patients.
Conclusion
Based on the complexity of the diagnostic evaluation process, biopsy results are subject to transposition, contamination, or mislabeling, therefore adopting a DNA time out for malignant cases is prudent. Since implementing the DSPA program for breast biopsies 18 months ago, our staff has helped ensure that we are making treatment recommendations with the most accurate information possible for our patients. With larger widespread adoption of DSPA programs, physicians can mitigate the risk of providing a patient with an inaccurate diagnosis and ensure that patients are receiving the quality of care they deserve. It’s the assurance that we, as physicians, should mandate for our patients—patients deserve that peace of mind.
Andrew Kenler is a board-certified general surgeon practicing at Bridgeport Hospital and an assistant clinical professor of surgery at Yale Medical School. He earned his medical degree from Cornell University Medical College in New York City and completed his surgery training at New England Deaconess Hospital, part of the Harvard Medical School system in Boston, Massachusetts. He is well known for his expertise in the use of image guidance (ultrasound and digital mammography) to diagnose and treat benign and malignant breast disease and oncoplastic surgery of the breast. Kenler serves on Know Error’s speaking bureau and can be reached at akenler@charter.net.