Safety Enhancements Every Hospital Must Consider in Wake of Another Tragic Neuromuscular Blocker Event
By the Institute for Safe Medication Practices
Problem:
National news recently exposed details about a 2017 fatal medication error that happened at a large, prestigious hospital and led to the Centers for Medicare & Medicaid Services (CMS) briefly placing its Medicare reimbursement status in jeopardy. The hospital’s status was quickly restored following submission of a plan of correction to CMS. Upon ISMP’s awareness of the event, it became imperative to share the lessons learned from the fatal event so other healthcare providers can avoid a similar tragedy.
The details of the error that follow are from a CMS report. As the story unfolds, we hope you will see that this type of error could happen anywhere given current system vulnerabilities frequently found in hospitals, particularly when using automated dispensing cabinets (ADC). In fact, ISMP has observed many of the same system vulnerabilities in other hospitals, and they are frequently at the root of a variety of medication errors reported to the ISMP National Medication Errors Reporting Program (ISMP MERP). Make no mistake—this type of error could happen in your hospital, and it is crucial to take steps now to reduce the risk of a similarly tragic event.
The error
A patient was admitted to the neurology intensive care unit (NCU) with a headache and vision field loss in her left eye. Magnetic resonance imaging (MRI) confirmed an intraparenchymal hematoma of the brain, possibly related to a suspected mass behind it. Two days later, the patient’s physician entered an order to transfer the patient to a step-down unit. The patient was then transported to radiology for a full-body positron emission tomography (PET) scan. While a radiology technician was explaining the PET scan to the patient, she requested medication to help ease anxiety due to claustrophobia. The patient’s physician was contacted, and he entered two electronic orders. The first order was for VERSED (midazolam) 2 mg intravenously (IV), with instructions, “For PET scan, if first mg insufficient, can give 1-2 additional if needed.” (Note: Versed is no longer available as a brand of midazolam.) The physician then clarified the order by prescribing a one-time dose of Versed 1 mg IV prior to the PET scan. A pharmacist verified the orders within a few minutes.
According to the CMS report, a radiology technician called the patient’s primary nurse to ask if she could send a nurse to administer the IV Versed. The primary nurse asked if a radiology nurse could administer the IV Versed, but the technician said that the nurse was uncomfortable administering this drug and that the patient would need to be monitored. The primary nurse said she would send a nurse to radiology to administer the IV Versed. The technician then administered a radioactive tracer to the patient so the PET scan could be completed in an hour after tracer absorption.
Because the primary nurse was covering another nurse’s patients, she asked a help-all (resource/floater) registered nurse, who was also orienting a new nurse, to go to radiology to administer the IV Versed. The help-all nurse had been on her way to the emergency department (ED) to conduct a swallowing study but agreed to first administer the IV Versed to the patient in radiology. At the NCU ADC, the help-all nurse entered the first two letters of the drug name, “VE,” into a search field under the patient’s profile, which was still active pending transfer to the step-down unit. No medications populated the search results, although the ADC allowed searches using just the first few letters of a medication’s name. “Versed” was not found because the ADC defaulted to generic drug name searches. To search by brand names, the setting needed to be changed on the ADC screen.
When the help-all nurse could not find “Versed” on the list, she initiated an override setting, entered “VE” again into a search field, and selected the first medication that populated the results, which was the neuromuscular blocker vecuronium, not Versed. Because the override function had been engaged, a red-box warning on the screen noted that the medication should be associated with a STAT order. But the ADC opened, and the nurse removed a vial of the neuromuscular blocker, vecuronium bromide lyophilized powder, 10 mg (1 mg/mL when reconstituted with 10 mL), believing it was Versed. Although neuromuscular blockers were on the hospital’s list of high-alert medications, there were no specific precautions in place prior to removal of the drug via override.
While removing the vial from the ADC, the help-all nurse noticed that the medication was a powder and turned the vial over to read the reconstitution directions on the back of the label, never reading the actual drug name on the front of the vial label. She also did not recognize that Versed is available only in a liquid injectable form, not a powder requiring reconstitution. The CMS report noted that the nurse placed the vecuronium vial in a plastic bag along with two 10 mL normal saline flush syringes, alcohol pads, and a blunt-tip needle, and labeled the bag with a patient sticker and a handwritten note that said, “PET scan Versed 1-2 mg.” She then went to radiology to administer the medication.
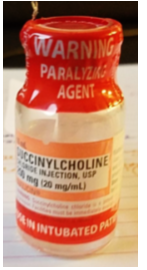
The patient had been moved into a radiology holding room to await tracer perfusion. The nurse found the patient, verified her identity, and told her that the medication would help her relax. She reconstituted the medication with 10 mL of 0.9% sodium chloride from the flush syringe and drew the reconstituted medication back into the flush syringe. During reconstitution, the nurse did not notice (or misunderstood) a warning on the red vecuronium vial ferrule that said, “WARNING: PARALYZING AGENT,” which has been previously overlooked or misunderstood with other neuromuscular blocker errors.
The help-all nurse administered an unknown quantity of IV vecuronium to the patient, believing it was Versed, and used the second 0.9% sodium chloride syringe to flush the patient’s IV line. The nurse thought she administered 1 mL (1 mg) of the drug; however, the empty vecuronium vial and the two flush syringes, both labeled 0.9% sodium chloride, were later brought back to the NCU to witness the wasting of what was thought to be Versed. One syringe had 8 mL remaining in it, and the other had 1.5 mL remaining. There was no way to determine which syringe contained the reconstituted vecuronium or how much drug the patient actually received.
The help-all nurse left the patient in the radiology holding room and went to the ED to conduct the swallowing test. She did not monitor the patient’s reaction to the medication, take vital signs, observe the patient for respiratory sufficiency, or determine if the patient needed an additional dose of what was thought to be Versed. Moderate sedation agents, including Versed, were not on the hospital’s list of high-alert medications. Although policies on conscious and moderate sedation were in place, the hospital’s drug administration policy did not specify the manner and frequency of physical assessment and monitoring of patients during and after drug administration. A radiology technician periodically observed the patient in the holding room via a camera. Because the patient’s eyes were closed, the technician assumed the patient was relaxing or bothered by the lights in the room. The camera was not sharp enough to visualize that the patient’s chest was not rising and falling. About 25 to 30 minutes after the vecuronium was administered in error, a transporter noticed that the patient was unresponsive. The patient was found pulseless and breathless, and a rapid response team/code blue was called. The patient was intubated and eventually regained spontaneous circulation.
The help-all nurse responded to radiology when the rapid response team was called and transferred the patient back to the NCU after resuscitation. She told the patient’s physicians that she had administered IV Versed to the patient about 30 minutes before the code was called. She also handed the bag containing the empty vial and syringes with leftover drug to the patient’s primary nurse to document the waste. The primary nurse noticed the error, which was reported to the patient’s physicians and disclosed to the patient’s family.
After a few hours, the patient began displaying myo-clonic jerks and posturing consistent with anoxic brain injury. Computed tomography of the head showed some increase in swelling, but the area of bleed had not worsened. It was suspected that the medication error, not worsening hemorrhage, was responsible for the patient’s arrest and subsequent anoxic brain injury. By the next day, the patient’s neurological sequelae had worsened, and she died after life support was withdrawn.
Safe practice recommendations:
This fatal error involved accidental administration of a neuromuscular blocker to an unventilated patient by a practitioner who thought she was administering a different drug—an all-too-common scenario with errors involving neuromuscular blockers. We urge healthcare providers, ADC vendors, drug manufacturers, and regulatory/standards–setting agencies to implement the following recommendations, as applicable, many of which address the causal factors in this tragic event. Most of the recommended strategies can help reduce the risk of an error when removing certain facility-designated medications from an ADC, including via override. Many of these recommendations are summarized in Table 1. For additional strategies critical to the safe prescribing, storage, selection, preparation, and administration of neuromuscular blockers, please refer to our June 16, 2016 newsletter article, “Paralyzed by Mistakes—Reassess the Safety of Neuromuscular Blockers in Your Facility” (www.ismp.org/node/247).
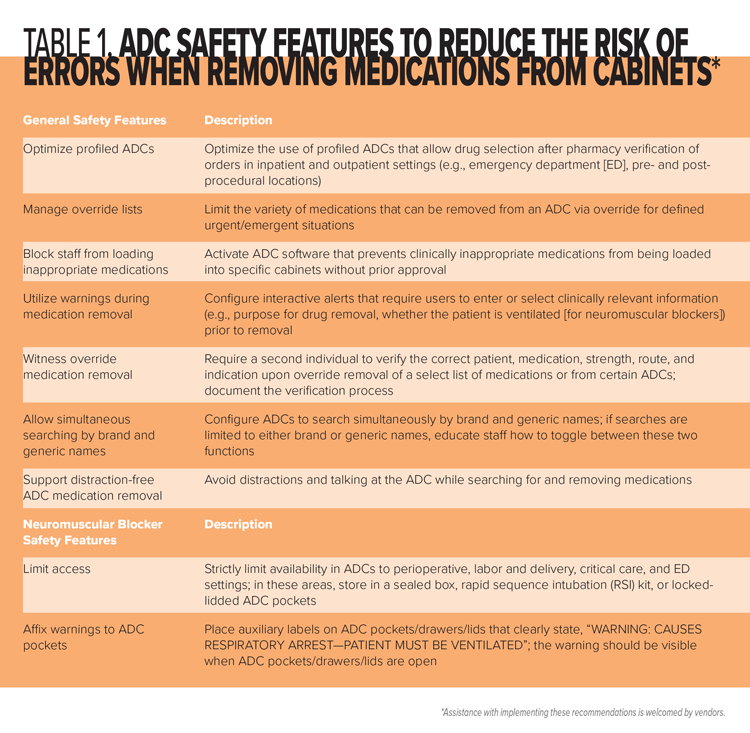
Recommendations for Healthcare Providers
Plan for sedation. Establish a standard process for patients who require sedation prior to radiology procedures due to claustrophobia, that starts with an oral anxiolytic (e.g., LORazepam) as the medication of choice, when appropriate, and includes patient monitoring requirements during and after drug administration.
Include IV moderate sedation agents on high-alert medication lists. Include medications commonly used for moderate sedation (e.g., IV midazolam) on the hospital’s list of high-alert medications and implement risk-reduction strategies to prevent errors and patient harm with these medications. For example, administration of IV medications commonly used for moderate sedation should require specific patient monitoring, regardless of the setting in which the sedation occurs or its intended use (e.g., anxiety vs. moderate sedation).
Store neuromuscular blockers safely. Eliminate the storage of neuromuscular blockers in areas where they are not routinely needed. In patient care areas outside perioperative settings where they are needed (e.g., critical care, ED), provide neuromuscular blockers in a sealed box or rapid sequence intubation (RSI) kit. For ADCs in areas not authorized to stock neuromuscular blockers, enable the ADC block-load feature, if available, to prevent users from inappropriately stocking the cabinet with these high-alert medications. If vials must be stored in ADCs, keep them in locked-lidded pockets.
Affix warnings. Place auxiliary labels on all storage locations and/or ADC pockets/drawers/lids that contain neuromuscular blockers that clearly warn that respiratory paralysis will occur, and ventilation is required (e.g., “WARNING: CAUSES RESPIRATORY ARREST—PATIENT MUST BE VENTILATED”). The warning should be visible when ADC pockets/drawers/lids are open. As an alternative (or in addition) to labeling storage bins and/or ADC pockets/drawers/lids, affix an auxiliary warning label directly on all vials and other containers. Be aware that the use of a shrink wrap sleeve (see below) for this purpose on different neuromuscular blockers can make them look similar and contribute to mix-ups. Limiting the variety of neuromuscular blockers available in ADCs can help reduce similar appearance.
Build interactive warnings. Display an interactive warning (e.g., “Patient must be intubated to receive this medication”) on ADC screens that interrupts all attempts to remove a neuromuscular blocker via a patient’s profile or on override. The warning should require the user to enter or select the purpose of the medication removal (“other” should not be a choice) and verify that the patient is (or will be) manually or mechanically ventilated. This type of warning provides an opportunity to specify why the user is being interrupted and requires the user to document a response. However, it may not be possible to block access to the medication based on the response. In most ADC systems, this type of warning is configurable by medication, and in some systems, by cabinet.
Clarify override policies. Review the hospital’s ADC override policy to confirm its permitted use is limited to emergency or urgent situations when a patient would be significantly compromised by the delay that would result from pharmacy review (or if a licensed independent practitioner controls the medication use process). Be sure the policy clearly communicates the hospital’s overall expectation of very limited overrides for urgent and emergent situations that are defined (e.g., antidotes, rescue agents, reversal agents, life-saving medications, comfort care medications for acute pain and intractable vomiting), along with an explanation of the safety risks of removing and administering a medication via override before pharmacy verifies the order.
Avoid unjustifiable overrides. Overrides should be limited to a handful of medications and can be configured by medication, user, and cabinet. Many high-alert medications should not be on an override list. However, neuromuscular blockers may be needed via override for emergency intubation. Nevertheless, if neuromuscular blockers are on a list of overridable medications, each override should be situation-dependent and justifiable, and not based alone on its availability on a list of overridable medications.
Require a witness upon removal of certain medications on override. Provide an automated prompt and require documentation of an independent double-check with another practitioner at the ADC when removing specific facility-defined medications via override. The second practitioner should verify the correct patient, medication, strength, route, and indication (against the electronic order when available). “Witness on dispense” by cabinet and by drug is an available prompt with some ADC systems that also allows documentation of the verification process.
Implement barcode scanning verification in all areas. Prior to administration, verify each medication via barcode medication administration (BCMA) to ensure accuracy.
Require patient monitoring. Patients who receive sedation for procedures (e.g., IV midazolam) require some level of monitoring, regardless of the indication. Hospital procedures should specify required monitoring, including use of pulse oximetry and other means of evaluating the adequacy of ventilation, along with criteria for when monitoring can be stopped. Monitoring requirements should be approved by the anesthesia department to standardize the care of patients who receive IV sedation and provide oversight.
Avoid reconstitution using flush syringes. Do not dilute or reconstitute medications by drawing up the contents into a commercially available, prefilled 0.9% sodium chloride flush syringe. This results in the syringe, now containing medication, being mislabeled as 0.9% sodium chloride. Also, these syringes have not been approved by FDA for reconstitution, dilution, and/or subsequent administration of IV push medications.
Ensure distraction-free ADC drug removal. Avoid distractions and talking at the ADC while searching for and removing medications.
Educate staff. Teach practitioners to access and remove medications in profile mode whenever possible, as it directs them to a patient-specific medication profile and limits their access to medications that were verified by a pharmacist. Be sure practitioners understand the safety risks with obtaining medications via override and required safeguards for drugs removed via override. Also teach practitioners how to toggle between brand and generic name search functions if they are separate, and to verify the drug search criteria if initially unable to find the desired medication, rather than triggering an override. Be sure practitioners who administer procedural sedation (e.g., IV midazolam) are familiar with patient monitoring parameters.
Monitor overrides. Monitor ADC overrides daily to verify appropriateness, transcription of orders, and documentation of administration. Any overrides that are not appropriate or do not have corresponding medication orders require follow-up. Also review aggregate override usage reports monthly, trending by medication, user, and location, to assess appropriateness, determine how well the hospital is managing overrides, and address barriers to the pharmacists’ review of medication orders prior to drug removal.
Recommendations for ADC Vendors
Increase number of drug name letters required when searching. Current ADC systems allow users to enter just one letter (e.g., Omnicell® XT, AcuDose-Rx) or two letters (e.g., Pyxis™) of a drug name before populating a list of potential choices. Older software versions may require entry of more letters, but more recent changes were made to require fewer letters, sometimes to accommodate international language requirements. While efficiency is important, and spelling errors are a concern, safety may be jeopardized by allowing fewer than five letters of a drug name to populate the search results. (See item #19 in the ISMP Guidelines for Safe Electronic Communication of Medication Information: www.ismp.org/node/1322.) It is recommended that vendors review potential software changes to allow a configurable option for the required number of letters to narrow the choices, ideally to one drug or drug category. (BD/Pyxis has agreed to consider this revision, although the search would likely not require a set number of letters to be typed, but instead be dynamic, allowing only the necessary letters to be typed to isolate a single medication.) As an alternative, vendors could require a minimum of the first five letters of a drug name before populating search fields. (Omnicell has agreed to consider this revision for Omnicell XT and AcuDose-Rx.)
Alert users to generic/brand name searches. Some newer ADC systems allow both brand and generic drug names to be displayed and searched. Earlier versions may allow only one or the other. Vendors should enable simultaneous searching by both brand and generic drug name. If brand and generic search capabilities are separate, the ADC screen should clearly display to the user which type of search is currently being conducted (generic or brand) and make it easy to toggle between the two functionalities.
Recommendation for Manufacturers and Regulatory/Standards-Setting Agencies
Institute labeling changes. ISMP has discussed with FDA the need for overall improvements to neuromuscular blocker container labeling. While the caps and vial ferrules note “WARNING: Paralyzing Agent,” it’s clear that these warnings have not been consistently effective and may go unheeded, misunderstood, or missed altogether.
As a result, between March and November 2018, FDA requested manufacturers of neuromuscular blockers to revise the carton, container, and prescribing information to further promote safe use. These recommendations were partly influenced by publication of our June 16, 2016 newsletter article, Paralyzed by Mistakes—Reassess the Safety of Neuromuscular Blockers in Your Facility. Specifically, FDA asked manufacturers to add the statement, “WARNING: Paralyzing Agent” in red, bold font to the principal display panel on the carton and container label, directly below the strength. They also asked manufacturers to add a statement to the side panel in red, bold font: “WARNING: Paralyzing Agent. Causes Respiratory Arrest. Facilities must be immediately available for artificial respiration.” Warnings about inadvertent administration to patients for whom the drug was not intended, and the catastrophic outcomes, were also requested in the prescribing information under the WARNINGS section, and a recommendation to store neuromuscular blocker vials with the cap and ferrule intact (which carries a warning) to help prevent errors was also requested in the DOSAGE AND ADMINISTRATION section. Look for these labeling changes soon.
*ISMP greatly appreciates the cooperation of BD/Pyxis and Omnicell for providing us with information about available cabinet configurations that could be utilized and may be considered in the future to prevent product selection errors.
From the January 17, 2019 edition of the ISMP Medication Safety Alert!
