Proceedings from the Quality Colloquium: Six Sigma Approach. Reducing CVC-Related Bloodstream Infections
May / June 2006
Proceedings from the Quality Colloquium
Six Sigma Approach
Reducing CVC-Related Bloodstream Infections
Central venous catheters (CVC) are appropriate and life-saving interventions for critically ill patients to ensure venous access to provide fluids, medications, and nutrition, as well as hemodynamic monitoring. The use of CVCs also places the patient at high risk for a bloodstream infection (BSI). Published rates for mortality in patients with central line related BSI “range from no increase in studies that controlled for severity of illness to a 35% increase in mortality in prospective studies that did not use this control” (CDC, 2002, p. 3). Treatment costs associated with BSI may range from $34,508 to as much as $56,000. However, BSI is also preventable. Guidelines for the Prevention of Intravascular Catheter-Related Infection, published by the Centers for Disease Control (CDC) and Prevention in August 2002, provides a detailed plan for prevention. The Institute for Healthcare Improvement (IHI) also includes prevention of central line associated infection as a bundle in the 100,000 Lives Campaign.
Florida Hospital is a 1,800 bed healthcare system with seven campuses covering three counties in central Florida. Our organization monitors the BSI rate specific to adult and pediatric critical care units and the overall BSI rate for all units system-wide. The critical care BSI rate is determined by dividing the number of central line associated BSIs by the number of central line days multiplied by 1,000. The internal benchmark is the aggregate top quartile specific to the critical care type as determined by National Nosocomial Infections Surveillance (NNIS) System. The overall BSI rate is calculated by dividing the number of central line associated BSIs by patient days multiplied by 1,000. Patient days are used for overall BSI calculation as device days are not tracked for all units. Based on the control chart and trending, the internal benchmark is adjusted downward annually in our journey for prevention.
The baseline metrics for this Six Sigma project were the overall 2003 BSI rate and the number of BSI cases secondary to CVCs in 2003. Efforts to reduce BSI in 2003 included a policy banning artificial nails for direct care providers in February, conversion to 2% chlorhexadine skin prep on supply carts in June, and BSI staff education in November. As a result, the overall BSI rate decreased by more than 40% in 2004. The decision to apply Six Sigma methodology was viewed as an opportunity to continue the journey to prevent BSI. The cross functional team was led by a Black Belt under the mentorship of a Master Black Belt and included an infectious disease physician, infection control practitioners, a nurse manager, nursing educators, front line nursing staff, an intravenous access nurse, a laboratory technician and a nurse from materials management. Use of Six Sigma methodology provided an educational opportunity for team members who were not familiar with the define, measure, analyze, improve, control (DMAIC) process.
Define
The defined scope of the project included all inpatients older than 17 years, system-wide, who had a positive blood culture within 48 hours of admission or re-admission within 2 weeks with a confirmed CVC-related BSI based on the CDC definition. Patients with BSI secondary to tunneled lines, port line, dialysis lines, peripherally inserted central catheter (PICC) lines, or peripheral lines were excluded. Based on historical data, 43% of BSI infections were in scope of the initiative.
Measure
The baseline monthly average was 6.33 cases of CVC-related BSI. A 20% reduction goal was established for 2005, which translated to a target of 5 or fewer cases per month or 16 annually. Baseline measurement of variable cost and average length of stay (ALOS) was determined by a comparison of diagnosis-related groups (DRGs) for patients with confirmed central line-related BSI with a patient population of identical DRGs who did not have BSI. Using this metric, the variable cost was $16,699 more for patients with BSI, and the LOS was 20.6 days longer than the non-BSI population. Baseline mortality rate for the patient population was 13%.
Analyze
Analysis of the overall BSI baseline rate demonstrated normal distribution with a pvalue of 0.308. The process was in control with no special cause variation and a sigma of zero. The team developed a supplier, input, process, output customer (SIPOC) flow chart, which is a high-level process map. Drivers or Xs were identified by the team’s cause-and-effect fishbone diagram. The team used the fishbone diagram to identify the critical Xs of focus for this initiative. Critical Xs included hand hygiene, maximal barrier precautions, skin prep, and an antimicrobial catheter. Hand hygiene, sterile barriers, and 2% chlorhexadine align with the CDC guidelines and are ranked as Category IA, which indicates the highest evidence-based research. This also aligns with the IHI central line bundle. Antimicrobial catheters are a Category IB CDC recommendation, which means a strong recommendation with supportive clinical evidence to indicate a reduction in BSI.
A stakeholder analysis indicated a high level of support by administration, the Infection Control Committee, infectious disease (ID) specialists, critical care intensivists, advanced registered nurse practioners, and clinical nurse specialists. The team ID physician authored an educational article for the physician newsletter that focused on the critical Xs. The team also authored a companion article for the nursing newsletter. A Gauge Repeatability and Reproducibility (R&R) assessment was done to measure variation in infection control findings. The results demonstrated 100% accuracy for repeatability and 90% accuracy for reproducibility for infection control surveillance by the infection control practitioners. The infection control department has now incorporated the Gauge R&R to audit accuracy for new employees and annually for all employees.
Improve
The decision for a custom tray for CVC lines was approved through the Infection Control Committee. The tray includes maximal barrier precautions, 2% chlorhexadine with alcohol skin prep, and an antimicrobial catheter for use in the emergency department (ED) and all inpatient units. The anesthesiology department opted to omit sterile barriers based on the rationale of cost containment, as sterile barriers are available in the operating room suite. The process to convert all trays began in May 2004 and was completed in November 2005. A system-wide hand hygiene campaign was introduced in December 2004. The rollout included an employee poster contest as a strategy to help secure stakeholder buy in and influence. Top winners received prizes and the posters were professionally reproduced and distributed through the marketing department.
Reports that trays without sterile barriers were inadvertently distributed to ED and inpatient units were a concern because sterile barrier supplies are not readily available in these areas. As a result, physicians were occasionally using non-sterile gowns intended for isolation. This discovery prompted the development of standard operating procedures (SOPs) for tray distribution. The SOPs were disseminated to each campus. Unfortunately, this did not resolve the issue since the trays are very similar in appearance. The next level intervention recommended by the team was error proofing the trays by placing sterile barriers in all custom trays. This has been endorsed by administration and is currently in deployment. Serendipitously, this is also cost-effective, as the sterile garb placed in the custom trays is less expensive than sterile garb used for surgical procedures in the OR.
The target goal to reduce the number of CVC-related BSI cases by 20% or 16 annually for 2005 was achieved by June 2005 (Figure 1). Based on the baseline 13% mortality rate, a reduction of 16 cases represents the prevention of 2 deaths. Using a per diem room cost, the finance department conservatively estimates savings at $318,029 for the first two quarters. Our internal analysis demonstrated an average of $16,699 additional variable costs per case which would equate to a savings of $271,984. The overall BSI rate is well below the initial target, which has since been reduced to reflect progress. An action plan was deployed in May 2005 to address an increase number of BSI specific to other lines outside the scope of this project. The Sigma score improved from zero to 1.8.
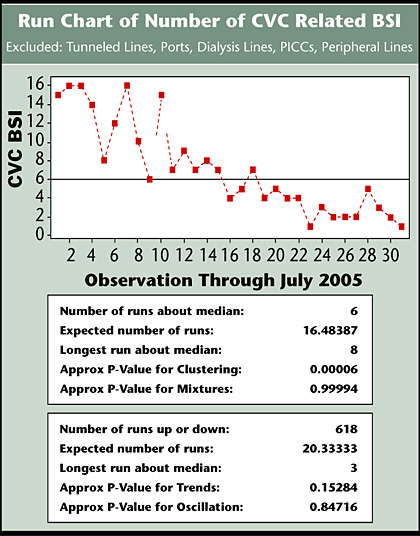
![]()
A new mean was established to reflect the reduction in the overall BSI rate from 2003 to 2004 (Figure 2). Although the process remains in control with no special cause variation, additional analysis revealed an increase in the overall rate from February to March. This was attributed to an increase in lines that had been excluded from the scope of the project. An action plan was fully deployed in May to address these issues.
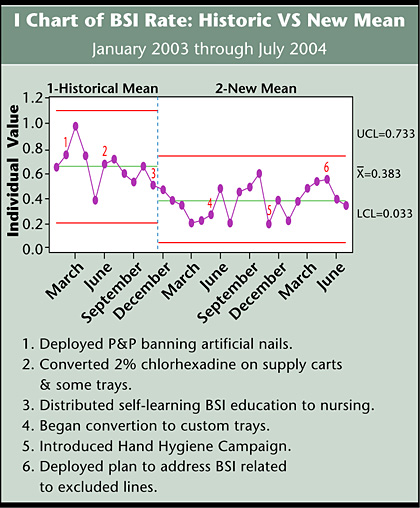
![]()
Control
The director for infection control and patient safety will assume accountability for measuring the monthly overall BSI rate based on the CDC definition. The number of CVC-related BSIs is extrapolated and tracked monthly with a continued target of five or fewer cases per month. The finance department is then notified of the number of monthly CVC-related BSI cases by campus. Estimated savings is calculated monthly and year-to-date by the finance department and reported to the process owners.
Summary
The Six Sigma approach to reduction of the BSI rate has proven successful, but the journey continues. Error proofing of trays is in process. The new error-proofed trays will also include a checklist to monitor sterile technique for insertions as recommended by IHI. A process proposed by a critical care intensivist for granting new and continued physician privileges to insert CVC lines is currently going through the approval channels. Options for credentialing include a documented training pathway, documentation of practice of a specific number of insertions annually or by examination. Additionally a new safety professional practice model track is being developed for RNs and LPNs. Nurses who apply for this track will heighten awareness and surveillance for prevention of all patient safety issues. Our organization has also committed to the IHI central line bundle. Policies and procedures have been revised to reflect appropriate catheter site and administration system care as well as no routine replacement. Nursing will become engaged by collaboratively monitoring continued need for a CVC.
Lois Yingling earned her MSN from the University of Florida and BSN from Akron University. She has an international certification as a Certified Professional in Healthcare Quality. Her current position is clinical best practice development coordinator for the clinical performance improvement department at Florida Hospital in Orlando, Florida. She received her Six Sigma Black Belt Training at Florida Hospital and continues to lead and mentor projects. This project was the subject of a poster at IHI’s National Forum in December 2005. Yingling may be contacted at Lois.Yingling@flhosp.org.
Bibliography
100k lives.Institute for Healthcare Improvement. www.ihi.org
Berenholtz, S., et al. (2004). Eliminating catheter-related bloodstream infections in the intensive care unit. Critical Care Medicine, 32(10).
CDC Guidelines for the Prevention of Intravascular Catheter-related Infections, MMWR. August 9, 2002; Volume 51, Number RR-10. www.cdc.gov/mmwr/
Hu, K., et al. (2004). Cost-effectiveness of maximal sterile barriers. Clinical Infectious Diseases, 39(15).
National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004. (2004). American Journal of Infection Control 32, 470-485.
