Peripheral IVs: Fewer and Better
Peripheral IVs: Fewer and Better
Replacing PIVs only when clinically indicated offers many advantages.
By Michelle DeVries, MPH, CIC
Our hospitals are busy, high-tech places for healing. Every member of the healthcare team is involved in a critical mission to provide the best possible patient care or support service to help achieve that goal. The healthcare industry is also increasingly regulated and transparent. Potential customers can go online and quickly find patient safety indicators reported by individual institutions, often through state as well as federal initiatives. Healthcare associated infections—central line-associated bloodstream infections, catheter-associated urinary tract infections, laboratory-identified MRSA in blood cultures, Clostridium difficile (C. diff.) events, and surgical infections following abdominal hysterectomies and colon surgeries—are among the reported outcomes (Centers for Medicare & Medicaid Services, 2014). Hospital performance on each of these is shared online along with a simple interpretation of this data. This structure creates an environment highly focused on meeting and exceeding certain outcomes. At the same time, that structure may distract attention away from worrisome issues that aren’t receiving the same public attention.
The majority of patients who enter hospitals receive a short peripheral catheter or peripheral intravenous line (PIV) at some point during their stay. The literature shows that somewhere between 70% and 80% of patients have at least one PIV during their hospitalization (Zingg & Pittet, 2009). These commonplace devices are the backbone of care delivered throughout inpatient units, yet they receive little or no attention from patient safety or infection prevention, overshadowed by concerns about mandated reporting of infections related to central lines. For an exhausted bedside nurse caring for a ventilated patient who has a central line as well as a peripheral line, foley catheter, and is perhaps on isolation precautions, there are seemingly countless tasks required to help deliver the patient’s treatment plan safely. Each device brings great opportunity for healing, but it also may put the patient at risk of developing a healthcare-associated infection (HAI). The seemingly humble PIV’s contribution to this or other patient complications is often overlooked. The population of patients exposed to PIVs is a huge un-tapped opportunity for a true “everyone wins” scenario.
 |
|
|
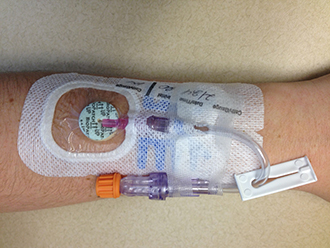
Figure 1. Peripheral IV catheter with intraluminal and extraluminal protection applied in conjunction with securement dressing. Courtesy of Ethicon® Biopatch® Products |
Most people have experienced a PIV cannulation at some point in their lives and likely recall it as an unpleasant, painful procedure. Furthermore, Kokotis (2005) reported that PIVs are placed successfully on the first attempt only 40% of the time; 27% of patients endure three or more “sticks” before a line is inserted successfully (Barton, Danek, Johns & Coons, 1998). In the United States, most hospitals still require their nurses to repeat the whole process again simply because 72 to 96 hours have gone by, even if the line is functioning well and has no signs of complications.
In 2010, however, Webster, Osborne, Rickard, and Hall published a Cochrane analysis asking a key question: Why are we putting our patients through PIV placement every three to four days? They concluded that there was no benefit in terms of infection or phlebitis from routinely rotating PIV sites versus leaving them in until no longer clinically indicated Webster et al. updated their study in 2013, reviewing seven new studies that encompassed almost 5,000 more patients and drew the same conclusion. This time, they also stratified their findings between continuous and intermittent infusion and still they could find no differences in phlebitis or bacteremia to justify putting patients through an IV restart simply because a pre-defined amount of time had passed (Webster et al., 2013).
At the time of the first study, the CDC’s guidance admonished clinicians to change PIVs at least every 72 to 96 hours. Even with the data from the Cochrane review, few hospitals wanted to be in the position of making patient care decisions directly in conflict with CDC. In 2011, the CDC issued updated guidelines and addressed this same issue with a different recommendation. That guidance, which is still in effect, now advises that PIVs should be changed “no more frequently” than every 72 to 96 hours (O’Grady et al., 2011). Those few words present a completely different opportunity. Also in 2011, the Infusion Nursing Society (INS) updated its Standards of Care. INS went even a step further and removed all reference to time from their dwell-time recommendation, allowing PIVs (consistent with both Cochrane reviews) to remain in until no longer clinically indicated (Infusion Nurses Society, 2011). One more major publication also came out on this topic. In Lancet, Rickard et al. (2012) found that updating PIV policies to extend dwell time to clinical indication can save nursing time (about 15 minutes), supply costs, and decrease the number of procedures a patient must go through. The new policy also saved one unnecessary restart for every five patients (Rickard et al., 2012).
PIVs and Risk of Infection
I am an infection preventionist and have always conducted surveillance on all laboratory-confirmed bloodstream infections in my hospitals, not just CLABSIs, so I know PIVs carry a certain amount of risk (DeVries & Mancos, 2012). Infection rates for PIVs are lower than for CLABSIs, but PIV utilization may be four times higher than central line utilization, so the total number of infections is not insignificant (Wischnewski et al., 1998).
A study by Trinh and colleagues reviewed PIV bloodstream infections specifically caused by Staphylococcus aureus (S. aureus). During 33 months of retrospective review, they identified 24 cases, including two deaths and a transfer to hospice. (Trinh et al., 2011). Unfortunately, the majority of infection prevention and control programs do not currently include this type of surveillance. This is something that may change, particularly as clinical indication begins to become the norm.
In 2014, INS came out with a Short Peripheral Catheter Checklist. Among the recommendations is to develop surveillance systems for infections (among other complications) with these devices. Similarly, this year, SHEA/IDSA updated their CLABSI compendium document. While the focus remains on central line-associated infections, they introduce the concept that infections do occur from short peripheral catheters and raise the possibility that surveillance may need to be expanded to include these devices at some point (Marschall et al., 2014)
Moving Toward Clinical Indication
As I read more research and began networking within my own institution to move toward clinical indication, I became increasingly convinced that this initiative would have no downside if we took the time to plan our strategy carefully, including staff education and product selections. Patients are spared needless, painful IV sticks; the first PIV placed during admission could possibly be their last during that stay. With the right scripting, we have the potential to help drive patient satisfaction by helping them understand that this change is for their benefit.
 |
|
|
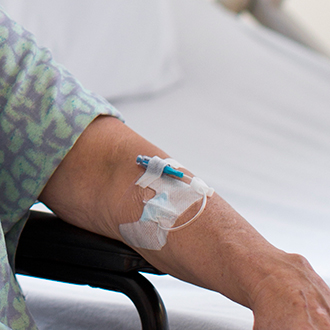
Figure 2. Peripheral IV catheter with integrated stabilization platform and extension set. Photo courtesy of BD Medical |
With an aging population requiring frequent admissions, fewer IV starts can also help contribute to vessel preservation. This important consideration is frequently overlooked but has real benefits for individual patients on future admissions. For bedside nurses, the time saved by not having to arbitrarily restart PIVs can be redirected to focusing on site assessment of the devices or any other multitude of tasks with competing priorities. Although many nurses pride themselves on their excellent IV-start skills, they will agree that sparing the patient the procedure is a great goal.
From the supply chain side, fewer IV starts mean fewer IV start kits. That may free-up dollars to ensure that the best products are selected when developing kits to support the institution’s desire to extend the dwell time of these catheters. From the administration’s side, although PIV bloodstream infections are not currently part of federal reporting or value-based purchasing metrics, they certainly can influence patient satisfaction. Additionally, several studies report S. aureus as among the most frequent pathogen isolated in bloodstream infections associated with PIVs (Trinh et al., 2011). If the infection happens to be a resistant strain (methicillin-resistant Staphylococcus aureus [MRSA]), it is a reportable outcome to CMS. If treating that infection leads to development of C. diff, that too is reportable. The costs for treating those outcomes also contribute to what is known as the efficiency domain: Medicare spending per beneficiary (Centers for Disease Control and Prevention, 2014).
Using evidence-based guidelines, taking the time to plan staff education, and selecting a bundle of products to support your objectives will benefit the patient, staff members, and the hospital’s bottom line. It is well worth the time to learn how to make this happen in your institution. Our journey started with a careful review of both the CDC guidelines and INS standards to identify gaps between our current policies and practices and the details in those documents. Based on that, we chose to add sterile gloves to our start kits to help our staff comply with the recommendations from both publications to ensure sterile gloves are worn if the site is repalpated after skin prep is performed (O’Grady et al., 2011; Infusion Nurses Society, 2011). Similarly, we added a securement dressing to our bundle knowing that good securement could help meet our objective of allowing extended dwell times. We decided, however, that the tape was not going to be sufficient. After reviewing the evidence for preventing infections in central lines, we opted to add a CHG-impregnated sponge dressing—with an FDA-cleared indication to reduce bloodstream infections—to our inpatient kits to address concerns about bacterial regrowth under transparent dressings left in place for up to one week. Consistent with the latest data in the SHEA/IDSA document, we continued our practice of using alcohol-impregnated caps on all of our lines as well to reduce the risk on intraluminal protection (Marschall et al., 2014). Consistent with INS standards to reduce add-on devices and reduce manipulation, we utilize an integrated, closed IV catheter system.
We launched our new policy with inservicing to our patient care leadership group to help them understand the “why” behind the new policy. Bedside nurses started with CEU classes covering IV basics followed by team rounding to train all shifts on our new bundle to help ensure they understand the synergy of the choices we made as well as the benefit for themselves as well as the patients. It took time to get all the necessary elements in place, but the results have been well worth the investment of time at the front end. It’s time to consider the evidence, move beyond “we’ve always done it this way” thinking, and take the next step to truly help improve our patients’ experience!
Chellie DeVries is senior infection control officer at Methodist
Hospitals in Gary, Indiana. She may be reached at mdevries@methodisthospitals.org.
References
Barton, A. J., Danek, G., Johns, P., & Coons, M. (1998). Improving patient outcomes through CQI: Vascular access planning. Journal of Nursing Care Quality, 13(2), 77-85.
Centers for Disease Control and Prevention. (2014, December). Healthcare facility HAI reporting requirements to CMS via NHSN—Current or proposed requirements. Retrieved from http://www.cdc.gov/nhsn/PDFs/CMS/CMS-Reporting-Requirements.pdf
Centers for Medicare & Medicaid Services. (2014 December 18). Hospital value-based purchasing. Retrieved January 7, 2015, from http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/hospital-value-based-purchasing/index.html?redirect=/hospital-value-based-purchasing/
DeVries, M., & Mancos, P. (2012, September). Non-central line related laboratory confirmed bloodstream infections. Poster presented at the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), San Francisco, CA.
Infusion Nurses Society. (2011). Infusion nursing standards of practice. Journal of Infusion Nursing, 34(1S).
Infusion Nurses Society. (2014). Short Peripheral Catheter (SPC) checklist. www.ins1.org.
Kokotis, K. (2005). Cost containment and infusion services. Journal of Infusion Nursing, 28(3S), S22-S32.
Marschall, J., Mermel, L. A., Fakih, M., Hadaway, L., Kallen, A., O’Grady, N. P.,…Yokoe, D. S. (2014, July). Strategies to prevent central line–associated bloodstream infections in acute care hospitals: 2014 update. Infection Control and hospital Epidemiology, 35(7), 753-771.
O’Grady, N.P., Alexander, M., Burns, L. A., Dellinger, E. P., Garland, J., Heard, S. O.…Healthcare Infection Control Practices Advisory Committee. (2011). Guidelines for the prevention of intravascular catheter-related infections. American Journal of Infection Control, 39(4 Suppl 1), S1-34. doi:10.1016/j.ajic.2011.01.003
Pujol, M., Hornero, A., Saballs, M., Argerich, M. J., Verdaguer, M., Cisnal, M.…Gudiol, F. (2007). Clinical epidemiology and outcomes of peripheral venous catheter-related bloodstream infections at a university-affiliated hospital. Journal of Hospital Infection, 67, 22-29.
Rickard, C. M., Webster, J., Wallis, M. C., Marsh, N., McGrail, M. R.,
French, V.,…Whitby, M. (2012, September 22). Routine versus clinically indicated replacement of peripheral intravenous catheters: A randomised controlled equivalence trial. Lancet, 380(9847),
1066-1074.
Trinh, T. T., Chan, P. A., Edwards, O., Hollenbeck, B., Huang, B., Burdick, N.,…Mermel, L. A. (2011). Peripheral venous catheter-related Staphylococcus aureus bacteremia. Infection Control and Hospital Epidemiology, 32(6), 579-583.
Webster, J., Osborne, S., Rickard, C. M., & New, K. (2013). Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No.: CD007798. DOI: 10.1002/14651858.CD007798.pub3.
Webster, J., Osborne, S., Rickard, C., & Hall, J. (2010). Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database of Systematic Reviews 2010, Issue 3. Art. No.: CD007798. DOI: 10.1002/14651858.CD007798.pub2.
Wischnewski, N., Kampf, G., Gastmeier, P., et al. (1998). Prevalence of primary bloodstream infections in representative German hospitals and their association with central and peripheral vascular catheters. Zentralbl Bakteriol, 287, 93-103.
Zingg, W., & Pittet, D. (2009). Peripheral venous catheters: An under-evaluated problem. International Journal of Antimicrobial Agents, 34(Suppl4), S38-42.
