Medication Safety: Averting Highest-Risk Errors Is First Priority
May / June 2005
Medication Safety
Averting Highest-Risk Errors Is First Priority
Not all medication errors are created equal. In efforts to improve patient safety, healthcare systems need to give first priority to averting the medication errors with the greatest potential for harm. By targeting efforts to avert such errors, hospitals can achieve the most rapid and significant impact on improving medication safety.
The Institute of Medicine report, To Err Is Human, focused national attention on the need to improve medication safety (IOM, 2000). Since then, technology advances have provided new tools and data to help clinicians, administrators, and risk managers target the medication errors associated with the highest risk of harm — intravenous (IV) medication errors (Eskew, et al. 2002; Hatcher, et al. 2004; Wilson & Sullivan, 2004; Williams & Maddox, 2005).
In this series of articles, Part I reviews the need for improved IV medication safety; comparative risk of IV versus oral medications; the “speed to impact” (costs, time, ease of implementation, and capabilities to prevent high-risk medication errors) of various medication safety technologies; development of “smart” infusion systems; and published results to date. Part II reviews nursing satisfaction, wireless networking, smart patient-controlled analgesia (PCA) with respiratory monitoring, best-practice improvements, and return on investment (ROI).
Risk of Harm: IV Versus Oral Medications
Only a few high-risk drugs, such as coumadin, sedatives, and some chemotherapy agents, are administered orally. A far greater number, including heparin, insulin, morphine, fentanyl, propofol, and midazolam (NCC MERP, 1998; USP MedMarx, 2001; Winterstein, et al., 2002), can be delivered intravenously. IV medications are associated with 54% of potential adverse drug events (ADEs) (Kaushal, et al. 2001),Ý56% of medication errors (Ross, et al., 2000), and 61% of the serious and life-threatening errors (D. W. Bates, personal communication, October 2001). The IV route of administration for medications often results in the most serious outcomes of medication errors (Hicks, et al., 2003).
General-purpose infusion pumps, which made modern IV therapy possible, increased the potential for error. While precisely delivering IV medications from a few drops to as much as one liter per hour to patients weighing anywhere from 600 g to more than 300 kg, until recently the pumps could not limit the doses of infused medications or be customized easily for specific patient populations (Eskew, et al., 2002).
The ever-increasing complexity of the nursing environment and the current nursing shortage further increase the possibility of error. Demands often exceed an individual’s capacity to function without error, even for highly experienced clinicians (Hatcher, et al., 2004). Unnecessary variability in drug concentrations, dosing units, and dosing limits used in different areas of a hospital further complicates infusion programming and increases the risk of harm (Bates, et al., 2005).
Examples of potentially fatal errors include programming an insulin infusion for a 67 kg adolescent at 7 units/kg/hr (dose ordered was 7 units/hr); mistakenly pressing a “0” in place of a decimal on a neonatal infusion, changing from 3.2 to 304 mL/hr; and entering the dose of heparin at 800 mL/hr rather than 800 units/hr (correct rate was 8 mL/hr). A nurse would never give 100 pills to a patient, but can all too easily program a general-purpose IV pump to deliver a comparatively massive overdose (Hatcher, et al., 2004, Thurman, et al., 2004).
Preventable ADEs are also costly: approximately $5,000 per PADE (1993 dollars), with projected annual costs for a 700-bed hospital of $2.8 million — excluding costs of injuries to patients, malpractice costs, cost of admissions due to ADEs, or litigation (Bates, et al., 1997). The Nebraska Medical Center found increased costs per ADE of $8,000 (Graham, 2004). A 2005 survey by the University HealthSystem Consortium revealed that settlements for injuries related to PCA errors ranged up to $750,000 (UHC, 2003).
None of the control and monitoring systems that exist for other aspects of the medication use process — e.g., computerized prescriber order entry (CPOE), robotic drug dispensing, pharmacy-controlled drug cabinet access, and barcode medication administration (BCMA) systems — addresses the problem of infusion-device programming errors (Eskew, et al., 2002), particularly verbal orders and the programming changes required for titrating medication (Hatcher, et al., 2004; Thurman, et al., 2004). In addition, none of these technologies provides comprehensive data for evidence-based continuous quality improvement (CQI) initiatives.
To meet the need for improved IV medication safety at the point of care, “smart technology” was developed to standardize IV infusion administration, reduce IV programming errors, and streamline processes for IV administration by forcing many functions (Bates, et al., 2005).
Development of “Smart” Infusion Technology
In the mid- to late-1990s, Cardinal Healtha selected the Vanderbilt University Medical Center and the Nebraska Medical Center to help in the development of a new infusion safety technology. The use of human factors engineering tools and techniques resulted in a modular infusion safety system that was introduced at the American Society of Health-System Pharmacists meeting in December 2001.
A point-of-care unit contains the computer “brain” that programs the infusions and contains the safety software. Lightweight, large-volume infusion modules, as well as syringe, patient-controlled analgesia (PCA), capnography (EtCO2), and pulse oximetry (SpO2) modules, can be attached to or removed from the brain as needed for each patient. A common user interface for all modules helps to reduce complexity and improves ease of use.
The safety software can also be added to some traditional infusion pumps. The VA San Diego Healthcare System added IV amedication safety software to existing infusion devicesb in nine areas of the hospital.
A hospital configures up to 10 profiles for specific patient care areas (e.g., med-surg, adult ICU). Profiles include customized operating parameters, programming options, and drug libraries. Clinician selection of a specific profile adjusts the performance characteristics of the device to meet the needs of that particular patient care area or patient type. Each drug library contains institution-specific parameters for up to 1,000 drugs, all of which can be customized. The drug library provides the best-practice guidelines for each medication. The software performs a final “test of reasonableness.” If programmed parameters are outside of pre-established limits, the software provides an alert that must be addressed before infusion can begin.
In the programming errors above, the software would not allow the patient’s weight to be used in programming an insulin infusion. The neonatal infusion would not exceed a very low rate limit (e.g., 20 mL/hr) without notification, and the heparin overdose would be prevented by a maximum dose limit, e.g., 2000 units/hr. All alerts and the clinician’s response, e.g., correcting the programming of heparin to the ordered 800 units/hr, are documented in CQI logs. CQI data can be used to assess current practices and safety levels, guide improvements to optimize care and reduce costs, and report averted errors to the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and other regulatory institutions (Hatcher, et al., 2004).
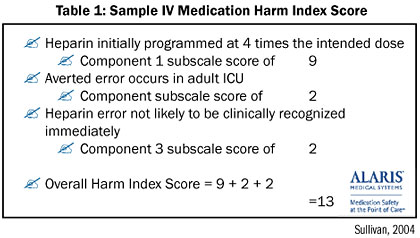
The IV Medication Harm Index (Sullivan, 2003) allows clinicians to objectively measure harm that has been averted through use of the system, based on drug risk/overdosing range, level of care/acuity, detectability of adverse event, and a summated score with a range of 3.5 to 14, with a higher score indicating greater risk of harm (Table 1).
ECRI published an independent analysis of commercially available smart pumps in Health Devices. ECRI has established the minimum standards and ideal criteria for defining dose error reduction systems (DERS) (ECRI, 2004). Health Devices has also published a comprehensive DERS checklist to assist hospitals in evaluating the various infusion devices and the level of safety they provide.
Speed to Impact
An IV medication safety system is associated with lower costs and more rapid implementation, compared with CPOE and BCMA (Wilson & Sullivan, 2004). Ease of implementation also is considered. Clarian Health Partners (1,400 beds) in Indianapolis, IN, was the first system in the United States to implement the systema on a hospital-wide basis. Development of a customized data set, including obtaining pharmacy and physician consensus among three hospitals, was completed in 60 days. Staff training included expert sessions and skills labs that combined hands-on exposure with an internet-based training module provided by the vendor. Of 2000 nurses, 97% completed the on-line simulation training, and 82% also attended hands-on in-service training sessions. The go-live installation in the first hospital was accomplished in three hours and required no additional FTEs (Eskew, et al., 2002).
Researchers at Brigham and Women’s Hospital (750 beds) in Boston conducted the earliest study of the efficacy of the new system in preventing medication errors (Rothschild, et al., 2005), while nursing evaluated various technologies for clinical use.
Initial research data from use of the earliest version of the software identified compliance issues. However, in practice, compliance improved to excellent following staff training that improved the “culture of use” and collaboration with the company that led to upgraded software with a more natural “mapping” of the IV infusion therapy that was easier to use. Following these changes, staff reported steadily improved compliance and excellent nursing acceptance.
After studying the issues for more than a year, Brigham and Women’s Hospital implemented the new IV safety system hospital-wide. Key deciding factors were the system’s speed to impact, error prevention, and platform for future safety innovations and wireless networking. Another very important consideration was the system’s data-collection capabilities, which would provide previously unavailable data that could allow staff to measure impact and identify process improvements (Brigham and Women’s Case Report, 2004).
Results
As hospitals are replacing their legacy infusion technologies, the smart infusion pumps are rapidly demonstrating their value in preventing IV pump programming errors and also identifying practice issues that previously were difficult to access.
Averted Programming Errors
Analysis of aggregated data from IV safety systems at seven institutions — community and regional hospitals, as well as major medical centers — shows that in an average 350-bed hospital, the system helps avert a potentially life-threatening IV programming overdose every 2.6 days, and an additional potentially significant IV error every 3.6 days (Cardinal Health, 2003).
Initial CQI data at Brigham and Women’s Hospital showed that the IV safety system helped avert potentially significant IV administration errors, e.g., 100 mg of morphine reprogrammed to 5 mg; heparin errors with extra zeros; 705 units of insulin reprogrammed to 7.5 units; and confusion between bolus, peripheral, and central infusion rates. Typical “close calls” included extra zeros, missing or misplaced decimal points, transposition of rate and dose (mg and mL), and 10- and 100-fold errors (Brigham and Women’s Case Report, 2004).
At Clarian Health Partners, a potentially life-threatening medication issue was averted during conversion to the system (Table 2). Initial CQI data showed that annual projections at Clarian for 940 IV safety systems included greater than 1 million infusion starts (potential opportunities for error), 18,500 alert messages, and 4,000 averted errors (Eskew, et al., 2002).

An 8-month study in three patient care areas representing 14,000 patient days at The Nebraska Medical Center (680 beds) showed 157 averted errors, with a potentially life-threatening dose initially programmed in 17 of these (Graham, 2004; Malashock, et al., 2004). Hospital administrators concluded that the incremental hospital costs of treating those errors would have significantly exceeded the cost of the IV medication safety systems (Graham, 2004).
Beta-testing at Vanderbilt University Medical Center (631 beds) documented 99 potential medication errors averted by the new safety system in 8 months, including some averted errors originally programmed at 20 times or greater the dosing limit (Hatcher, et al., 2004; Thurman, et al., 2004). CQI data on 20 continuous heparin infusions identified a potentially significant averted error in which the dose was originally programmed as 610 mL/hr and then, following the safety alert, was reprogrammed to 6.1 mL/hr (Wilson & Sullivan, 2004).
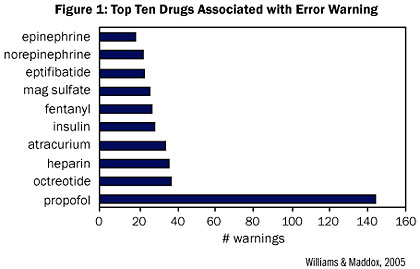
Figure 1 shows the top 10 drugs associated with safety alerts documented at St. Joseph’s/Candler Health System (675 beds) in Savannah, GA. Data for 100 systems from 8 patient care areas over 6 months documented 63 averted errors where researchers believed the new technology had a critical impact in preventing potentially serious infusion errors. Failure mode and effects analyses (FMEAs) showed nearly a four-fold reduction in risk related to setting IV heparin infusion rates as a result of the IV medication safety system, from a pre-implementation score of 210 to a post-implementation score of 56 (Williams & Maddox, 2005).
In the first six months of using the new system at Children’s Hospital and Health Center, San Diego (238 beds), approximately 15% of the alerts led to reprogramming, including averted errors that otherwise would have resulted in overdoses of high-alert medications at several times the dosing limits. Time-based data revealed that harm is not random, i.e., dosing errors occur in identifiable patterns, peaking significantly during busy times such as shift change, high admission volumes, and activities requiring drug distribution (Billman, 2004).
The smart IV systems are very effective in preventing programming errors. As illustrated by the above examples, however, not all alerts result in reprogramming. A high percentage of the alerts that are soft (i.e., can be overridden) result in confirmation that the dose exceeding the limit range is desired. This typically is a case where the best practices and the current practices are not in alignment. The Children’s Hospital data showing approximately 85% of the alerts resulting in overrides illustrates the value of the CQI data for clinical practice improvement. Hospitals employing smart infusion systems are bringing the current and best practices into closer alignment by using the data to measure and change practice. While not classified as errors in most cases, these alignment issues are nonetheless significant departures from accepted practice.
Conclusion
In targeting efforts and allocating resources to improve medication safety, hospitals should give priority to errors that pose the greatest risk of harm. Experience and CQI data from multiple hospitals — major medical centers, regional healthcare systems, and community hospitals — demonstrate the ability of an IV medication safety system to help prevent the most harmful medication errors and to provide actionable data for process improvements. Implementation of an IV medication safety system allows safety efforts to impact the highest-risk errors most rapidly and with the highest degree of success — namely, IV medications at the point of delivery to the patient (Williams & Maddox, 2005).
Part II in this series — nursing satisfaction, wireless networking, smart PCA, best-practice improvements, and ROI — will appear in the July/August issue of Patient Safety and Quality Healthcare.
Tim Vanderveen (tim.vanderveen@cardinal.com) is vice president of the Cardinal Health Center for Medication Safety and Clinical Improvement in San Diego. Prior to this position, Vanderveen was the director of clinical affairs, Medication Management Systems, for ALARIS Medical Systems. He joined IMED Corporation in 1983. In 1996, IVAC Medical Systems merged with IMED Corporation to form ALARIS Medical Systems, and in 2004 the company merged again with Cardinal Health, becoming the AlarisÆ Products business division of Cardinal Health.
From 1972 to1983 Vanderveen was on the faculty of the College of Pharmacy at Medical University of South Carolina and was director of the Division of Clinical Pharmacy.ÝHe also had a faculty appointment in the College of Medicine and was on staff at the Charleston VA Hospital. Vanderveen’s clinical practice was in nutritional support, and he co-founded one of the first multidisciplinary nutrition support teams. During his academic tenure, his interests were closely tied to drug therapy in patients receiving parenteral and enteral nutrition. Vanderveen received his BS and MS degrees from Purdue University School of Pharmacy and his PharmD degree from the Medical University of South Carolina.
References
Bates, D. W., Spell, N., Cullen, D. J., et al. (1997). The costs of adverse drug events in hospitalized patients. Journal of the American Medical Association 277, 307-11.Ý
Bates, D. W., Vanderveen, T., Seger, D. L., Yamaga, C. C., & Rothschild, J. (2005). Variability in intravenous medication practices: Implications for medication safety. Joint Commission Journal of Patient Safety and Quality 31(4), 203-210.
Billman, G. (2004 December). Evidence-based improvements prevent high-risk errors. Harm isn’t random. Nursing Management. (Suppl.), 7-8. JONA. 34:(Suppl),7-8.
Brigham and Women’s Hospital Case Report. (2004 November). Intravenous medication safety: immediate improvement and long-term vision. Retrieved May 3, 2005, from www.alarismed.com/na/press/resources.shtml.
Cardinal Health. (2003, March 13). Findings presented by Cardinal Health (formerly known as Alaris Medical Systems) to the National Patient Safety Foundation 5th Annual Congress.
ECRI. (2004 December) General purpose infusion pumps. Health Devices 33(12) 416-430.
Eskew, J. A., Jacobi, J., Buss, W., Warhurst, H., & DeBord, C. (2002). Using innovative technologies to set new safety standards for the infusion of intravenous medications. Hospital Pharmacist 37(11), 1179-1189.
Graham, J. (2004). The Nebraska Medical Center experience with smart infusion systems. In: P. J. Schneider (Ed.). Measuring medication safety with smart IV systems. HealthLeaders. 2004;7(12suppl):26-28.
Hatcher, I., Sullivan, M., Hutchinson, J., Thurman, S., & Gaffney, F. A. (2004). An intravenous medication safety system: preventing high-risk medication errors at the point of care. Journal of Nursing Administration 34(10), 437-439.
Hicks, R. W., Cousins, D. D., & Williams, R. L. (2003). Summary of information submitted to MEDMARX in the year 2002. The quest for quality. Rockville, MD: USP Center for the Advancement of Patient Safety.
Institute of Medicine (IOM). (2000). To err is human: building a safer health system. L.T. Kohn, J. M. Corrigan, & M. S. Donaldson (Eds.). Washington, DC: National Academy Press.
Kaushal, R., Bates, D. W., Landrigan, C., et al. (2001). Medication errors and adverse drug events in pediatric inpatients. Journal of the American Medical Association 285, 2114-2120.
Malashock, C.M., Shull, S.S., Gould, D.A. (2004 May). Effect of smart infusion pumps on medication errors related to infusion device programming. Hospital Pharmacy 39(5), 460-469.
National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). (1998). NCC MERP taxonomy of medication errors.
Ross, L. M., Wallace, J., & Paton, J. Y. (200). Medication errors in a paediatric teaching hospital in the UK: Five years operational experience. Archives of Diseases in Childhood 83, 492-497.
Rothschild, J.M., Keohane, C.A., Cook, E.F., Orav, E.J., Burdick, E, Thompson S, Hayes J, & Bates D.W. A controlled trial of smart infusion pumps to improve medication safety in critically ill patients.* Crit Care Med. 2005;33(3):533-540. (*See also pg 679.)
Sullivan, J. (2003). IV Medication Harm Index: results of a national consensus conference. In: Schneider P. J., (Ed.), Addressing harm with high-risk drug administration: Conference proceedings. San Diego, CA: The ALARISÆ Center for Medication Safety and Clinical Improvement, http://www.alarismed.com/alariscenter/pdf/1454BAddressingHarm.pdf (accessed April 17, 2005).
Thurman, S., Williams, M. A., & Gaffney, F. A. (2004 December). Intravenous medication safety systems help prevent harm and career-ending mistakes. Nursing Management. (Suppl), 2-4. JONA. 34:(Suppl),2-4.
University HealthSystem Consortium. (2003 June). UHC Patient Safety NetÅ finds PCA errors.
University HealthSystem Consortium. UHC Patient Safety NetÅ finds PCA errors.
USP MedMarx. (2001). Analysis of participating hospital data.
Williams, C., & Maddox, R. R. (2005). Implementation of an i.v. medication safety system. American Journal of Health-System Pharmacists 62, 530-536.
Wilson, K., & Sullivan, M. (2004). Preventing medication errors with smart infusion technology. American Journal of Health-System Pharmacists 61, 177-83.
Winterstein, A. G., Hatton, R. C., Gonzalez-Rothi, R., et al. (2002). Identifying clinically significant preventable adverse drug events through a hospital’s database of adverse drug reaction reports. American Journal of Health-System Pharmacists 59, 1742-9.
