Medication Safety – Averting Highest-Risk Errors Is First Priority Part II: Nursing Satisfaction,Wireless Networking,”Smart”Pain Management, Best Practice Improvements, and ROI
July / August 2005
Medication Safety
Averting Highest-Risk Errors Is First Priority
Part II: Nursing Satisfaction,Wireless Networking,”Smart”Pain Management, Best Practice Improvements, and ROI
Targeting medication safety efforts to give first priority to averting the highest-risk errors allows hospitals to achieve the most rapid and significant impact on improving medication safety. This series of articles describes technology advances that provide new tools and data to help clinicians, administrators, and risk managers avert the medication errors associated with the highest risk of harm — intravenous (IV) medication errors (Wilson & Sullivan, 2004; Williams & Maddox, 2005).
Part I in this series (May/June issue, PSQH) reviewed the need for improved IV medication safety; comparative risk of IV vs. oral medications; the “speed to impact” (costs, time, ease of implementation, and capabilities to prevent high-risk medication errors) of various medication safety technologies; development of “smart” infusion systems; and published results to date.
This article reviews nursing satisfaction and recent advances, including wireless networking, “smart” patient-controlled analgesia (PCA) with continuous respiratory monitoring, best practice improvements, and return on investment (ROI).
Nursing Satisfaction
Experience has shown that nursing acceptance is critical to the successful implementation and use of new technology. Nursing administrators at St. Joseph’s/Candler Health System, a 675-bed facility in Savannah, Georgia, believe that positive feedback regarding the IV medication safety systema resulted from the level of pre-implementation education of nursing and hospital staff coupled with the involvement of nursing in research and implementation of the technology. CQI data and regular checks using direct observation show 98% to 100% compliance for nurses using the safety software for programming. Implementation of the system was felt to demonstrate the hospital’s commitment to nurses and to give SJCHS an edge in nursing retention and recruitment (Fields & Peterman, 2005).
A survey conducted at The Nebraska Medical Center (680 beds) shows that over 90% of users said the IV safety system was “Easy” or “Somewhat Easy” to program and would “Agree” or “Strongly Agree” that the system provides a valuable safety net at the point of care (Graham, 2004). Results show that nurses strongly prefer using the infusion safety system over their previous IV pumps. Data also showed that use of the safety software prevented potentially fatal medication errors (Graham, 2004; Malashock, et al., 2004). Staff feels that infusion safety technology is needed to protect both patients and nurses against critical errors at the point of care (Graham, 2004).
Staff at City of Hope, one of the top 100 cancer centers, had a similar response. Nursing administrators felt that implementation of the IV safety system and the resulting CQI data on averted errors allowed cultural change to be introduced in incremental steps, as nurses saw the impact of this technology on medication errors (Steingass 2004).
Best Practice Improvements
The examples below are representative of best practice improvements that have been made as a result of infusion safety system implementation.
Sedation Use. During beta testing at Vanderbilt University Medical Center (631 beds), nurses reported that trauma patients transferred from the ICU often required a prolonged period to become responsive enough to start rehabilitative therapy.ÝAs a result of smart system CQI data analysis, “titrate to sedation” was replaced by titration to a specific scale. Staff education focused on using the scale to more closely monitor patient response.ÝAfter implementation of these changes, reports from nurses indicated decreased time between transfer and start of therapy, leading to decreased patient recovery time, decreased length of stay, and decreased potential for sedation-related complications (Hatcher, et al., 2004; Thurman, et al., 2004).
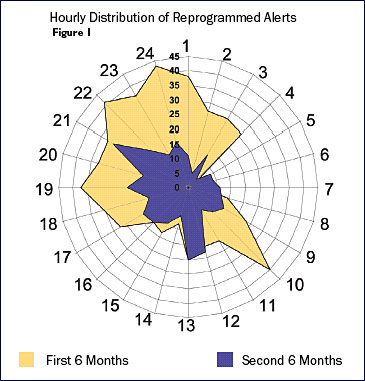
Staffing Effectiveness. At Children’s Hospital of San Diego, chronogram analysis (Figure 1) led to highly targeted practice improvement initiatives to address peak times for medication errors. Units were closed during shift change, and the timing of several elective activities was changed to reduce distractions. Since these changes, pump alerts are much less frequent and all of the former error peaks have been eliminated (Billman, 2004).
Improved Medication Protocol. Using CQI data from the safety software, staff at St. Joseph’s/Candler revised the heparin protocol to eliminate rate calculations by programming the dose (units/kg/hour) directly into the smart pump. This eliminated at least three steps in the medication process, multiple calculations, and multiple opportunities for error, thereby improving the safety and timeliness of heparin administration. The system allowed staff to discover that, if IV drug labels were reformulated to include the total volume and amount of drug, nursing staff would be able to program the system more easily and deliver the correct dose of medication (Williams & Maddox, 2005).
Return On Investment (ROI)
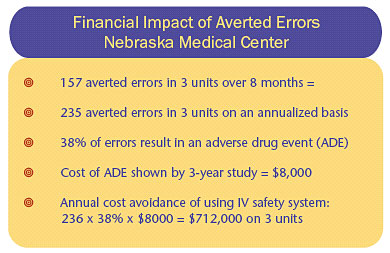
Analysis of reprogramming events documented in CQI logs at The Nebraska Medical Center for three nursing units involved in an 8-month study showed that an annual incremental expense that was avoided through use of the IV safety systems would be approximately $712,000 (Table 1) (Graham, 2004).
![]()
With three hospitals, 730 beds, 510 staff physicians, and gross annual revenue of $1 billion, Spartanburg Regional Health System (SRHS) is ranked as one of the top 100 most-wired hospitals in the United States. Implementation of 556 IV medication safety systems was accomplished in less than 6 weeks. Early CQI data indicated that the benefits of the system far outweighed any additional cost. Nurses, physicians, and pharmacists quickly saw the benefits of this technology. Error prevention data allows the hospital to demonstrate to the community their improvements in medication safety and commitment to quality patient care (Shingler, 2004).
Wireless Networking — “Smart” Infusion Systems
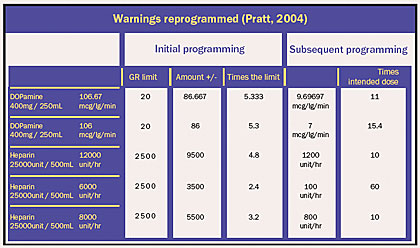
Wireless networking is one of several recent advances that have increased hospitals’ ability to use IV safety systems to improve medication safety. Wireless networks can link individual infusion devices to a central server, allowing CQI teams to obtain data for process improvement efforts and to update the software quickly. Networking can accelerate best practice and process improvements and provide insight into the usability of the equipment and the general practices associated with IV drug infusions (Pratt, 2004).
Sharp HealthCare in San Diego, California, installed IV infusion safety systems in five acute care hospitals totaling more than 1,400 beds, and then added wireless data transfer to optimize system use and maximize safety benefits. Analysis of CQI data shows that there were at least 13 harmful events avoided in 2 months at a single hospital (Table 2). Researchers felt that in any of these 13 cases, if the clinician had walked away after the infusion were started, there is little doubt the patient would have had an adverse event. Most events involved drugs that could cause clinically significant hemodynamic changes within minutes. Physiologic changes caused by heparin and insulin infusions could easily go undetected until they became clinically severe, for example, gastrointestinal hemorrhage or severe hypoglycemia. Feedback from the safety software prompting the clinician to make a correction avoided these adverse effects (Pratt, 2004).
![]()
Staff at Sharp has confidence that the IV safety systems with wireless connections have reduced harmful events associated with IV drug infusions and have provided tremendous insight into the administration of IV drugs. In addition, researchers note that unnecessary variations in treatment should be minimized and greater standardization should be used in IV medication therapy to improve patient safety. The safety software in the IV medication safety systems not only helps avert high-risk medication errors but also enables this standardization. Regular analysis of IV safety system data provides insight into avoiding harm, determining where learning needs exist with respect to IV medication safety, and to better understanding of current practices in the administration of continuous IV drug infusions (Pratt, 2004).
“Smart” Pain Management —
PCA with Continuous Respiratory Monitoring on One System Platform
PCA is an effective method of administrating high-risk narcotics. Nonetheless, analgesics-narcotics (Nebeker, 2005) and PCA therapy are associated with significant hazards (ISMP, 2002, 2003, 2005; JCAHO, 2004). MEDMARX and USP Medication Errors Report data from September 1, 1998, through August 31, 2003, shows that when PCA pumps are involved in medication errors, the chance for patient harm increases more than 3.5 times (USP, 2004). Smart systems that were previously available for general-purpose IV pumps now can provide the same functionalities for PCA. Averting PCA-related programming errors is important; however, with PCA, these are not the only potential cause of harm.
A major risk associated with PCA delivery of narcotics is the potential for respiratory depression from over-sedation. Individual response to opioids varies greatly. Even correctly programmed doses of opiates can suppress respiration and decrease heart rate and blood pressure (ISMP, 2003). In addition, factors such as “PCA by proxy” (doses administered by someone other than the patient), improper patient selection, and inadequate patient monitoring can also result in over-sedation (ISMP, 2003; JCAHO, 2004). Patients receiving PCA therapy may have undiagnosed co-morbidities such as obstructive sleep apnea. For these reasons, when using PCA, it is important to closely monitor not only device programming but also patient’s response to the therapy.
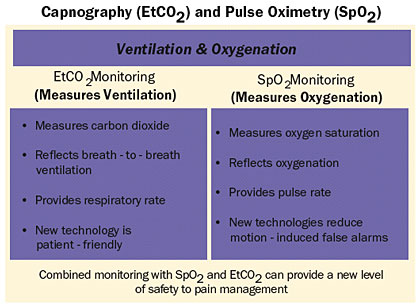
As shown in Table 3, continuous respiratory monitoring includes both pulse oximetry (SpO2) and capnography (EtCO2). The safety system includes pulse oximetry and capnography with PCA modules on a single platform with a common interface. Although earlier capnography devices were cumbersome and limited to the peri-operative and intensive care units, the newer technology is both user- and caregiver-friendly. As a result, capnography can now be used to monitor non-intubated patients in general nursing areas. When used with the PCA module, the safety system offers PCA/EtCO2 and PCA/SpO2 trending data at the bedside to assist clinicians in assessing respiratory response to PCA therapy.
![]()
St. Joseph’s/Candler was the first hospital system in the U.S. to implement the smart system.b In the first 3 weeks of use, the system helped SJCHS identify five patients who may have experienced undesirable clinical outcomes as a result of PCA therapy without continuous respiratory monitoring — three cases of previously unrecognized sleep apnea, one case of probable pulmonary embolus, and one case of early congestive heart failure. Experience has shown that despite care in programming of PCA devices and clinician second checks, a surprising number of errors were prevented in the first weeks of use. Continuous pulse oximetry and capnography may allow clinicians to identify undiagnosed conditions that predispose patients to respiratory complications from PCA narcotic delivery (Maddox & Williams, 2004).
PCA therapy is an effective method of titrating pain therapy but also potentially unforgiving if errors occur or patient status changes. The combination of best practice rules and use of continuous pulse oximetry and/or capnography monitoring reduces the risk of over-sedation and respiratory depression (Maddox & Williams, 2004).
Lessons Learned
Implementation of the IV safety system for continuous infusion, PCA, and respiratory monitoring has shown that at major medical centers, regional healthcare systems, and community hospitals:
- High-hazard drugs are indeed high hazards (ISMP, 2002, 2003; Billman, 2004; Pratt, 2004; Wilson & Sullivan, 2004; Williams & Maddox, 2005).

- Use of smart infusion systems in clinical practice has helped avert potentially serious drug administration errors (Billman, 2004; Pratt, 2004; Malaschock, 2004; Williams & Maddox, 2005) such as a 100-fold overdose of heparin (Wilson & Sullivan, 2004), as well as undesirable outcomes such as over-sedation detected by SpO2 and EtCO2 monitoring (Maddox, 2004), that could have been tragic and costly.

- With any new technology, a “culture of safety” and collaborative environment is needed to gain acceptance by users and for them to acquire comfort in its use (Williams & Maddox, 2004). Showing staff actual errors made at their institution can help increase awareness for the need to create a “culture of safety” and to avert high-risk medication errors.

- Humans can always defeat technology if it is perceived as a barrier. Use of human factors design principles to make devices user-friendly is critical (Leape, 2005).

- In selecting an infusion safety system, it is important to select a platform that can incorporate future developments. The process of implementing and using an infusion/information system is not a one-time “purchase” but requires an ongoing relationship between institution and vendor to continuously improve technology and best practices.
Quality Improvements
Smart IV systems can be the “canary in the mine” to identify where nursing workload issues exist and to target quality improvement to patient safety, best practices, and nursing satisfaction. These factors act synergistically and ideally position this medication safety system to function as an “information hub,” capable of integrating future innovative technologies and data — which holds the potential of further improvements in patient and medication safety (Billman, 2004).
Wireless networking and pain management systems, including PCA with capnography and pulse oximetry, further demonstrate the ability of “smart” infusion safety technology to help hospitals avert errors and undesirable outcomes and to quickly identify opportunities to improve best practices with regard to medications associated with the greatest risk of harm — IV medication errors at the point of care.
References
Billman, G. (2004, December). Evidence-based improvements prevent high-risk errors. Harm isn’t random. Nursing Management (Supplement). 7-8.
Fields, M., & Peterman, J. (2005) Intravenous medication safety system averts high-risk medication errors and provides actionable data. Nursing Administration Quarterly 29(1), 7786.
Graham, J. (2004). The Nebraska Medical Center experience with smart infusion systems. In P. J. Schneider (Ed.), Measuring medication safety with smart IV systems. HealthLeaders, 7 (12suppl), 26-28.
Hatcher, I., Sullivan, M., Hutchinson, J., Thurman, S., & Gaffney, F. A. (2004). An intravenous medication safety system: preventing high-risk medication errors at the point of care. Journal of Nursing Administration 34(10), 437-439.
ISMP Medication Safety Alert. (2002, May 29). More on avoiding opiate toxicity with PCA by proxy. Retrieved on June 16, 2005, from http://www.ismp.org/MSAarticles/pca.htm
ISMP Medication Safety Alert. (2003, July 10). Safety issues with patient-controlled analgesia Part I – How errors occur. Retrieved on June 16, 2005, from http://www.ismp.org/MSAarticles/issue.htm
ISMP Medication Safety Alert. (2005, January). Nurse Advise-ERR. Safety issues with patient-controlled analgesia. 3(1) 1-3.
JCAHO Sentinel Event Alert.(2004, December 20). Patient-controlled analgesia by proxy. Retrieved on June 16, 2005, from http://www.jcaho.org/about+us/news+letters/sentinel+event+alert/sea_33.htm
Leape, L. (2005). Editorial: Smart pumps: a cautionary tale of human factors engineering. Critical Care Medicine 33(3), 679.
Maddox, R. R., Williams, C. K., & Fields, M. (2004). Respiratory monitoring in patient-controlled analgesia. American Journal of Health-System Pharmacy 16,: 2628,2635.
Malashock, C. M., Shull, S. S., & Gould, D. A. (2004, May). Effect of smart infusion pumps on medication errors related to infusion device programming. Hospital Pharmacy 39(5), 460-469.
Nebeker, J. R., Hoffman, J. M., Weir, C. R., et al. (2005). High rates of adverse drug events in a highly computerized hospital. Archives of Internal Medicine 165, 1111-1116
Pratt, N. (2004). Intravenous medication safety wireless data system. In P. J. Schneider (Ed.), Measuring medication safety with smart IV systems. HealthLeaders 7(12 Suppl.), 39-44.
Shingler, R. A. (2004, December). Correcting risk of harm from IV medication errors: explore safety, financial, and community benefits. Nursing Management (Suppl.), 6-7.
Steingass, S. (2004). A framework for analysis of medication events using infusion safety CQI technology. In P. J. Schneider (Ed.), Measuring medication safety with smart IV systems. HealthLeaders 7(12 Suppl.), 21-25.
Thurman, S., Williams, M. A., & Gaffney, F. A. (2004, December). Intravenous medication safety systems help prevent harm and career-ending mistakes. Nursing Management. (Suppl), 2-4, Journal of Nursing Admnistration 34, (Suppl.), 2-4.
USP. (2004, September). Patient-controlled analgesia pumps. USP Quality Review. No. 81.
Williams, C., Maddox, R. R. Implementation of an i.v. medication safety system. American Journal of Health-System Pharmacists 62, 530-536.
Wilson, K., Sullivan, M. (2004). Preventing medication errors with smart infusion technology. American Journal of Health-System Pharmacists 61, 177-83.
