Making the Most of Data for Patient Safety: How Dose-tracking Software, a Customized Drug Library, and Expert, In-depth Analysis Provide Safer, Data-driven Dosing.
May / June 2007
Making the Most of Data for Patient Safety
How Dose-tracking Software, a Customized Drug Library, and Expert, In-depth Analysis Provide Safer, Data-driven Dosing.
Lutheran Medical experienced a concrete reduction in infusion-related medication events after implementation of the smart-pump technology and evidence-based protocols; from a total of seven events in 2005 to only three in 2006 — a 57% decrease.
In its report Preventing Medication Errors (2006), the Institute of Medicine estimated hospital patients are subjected to an average of at least one medication administration error per day. Two-thirds of preventable deaths due to infusion therapy within the hospital setting are attributable to manual programming errors with infusion pumps. Lutheran Medical Center, a 476-bed hospital in Brooklyn, New York, decided to address the threat of IV medication dosing events through an institution-wide infusion pump technology upgrade. Through implementing a customized drug library with “smart pump” dose limiting technology, Lutheran Medical Center witnessed a clear path toward a safer future for our patients.
The Institute for Safe Medication Practices and other safety organizations recommend smart-pump technology and double-check systems to reduce potential errors associated with manual programming of infusion pumps (AHA & ISMP, 2002). Lutheran Medical Center took patient safety one step further. Error reduction in itself is necessary, but identifying the most common event types is valuable to system-wide improvement. As such, we created a customized drug library specific to our institution’s needs and installed new software that would allow us to evaluate, for the first time, outcome data and trends associated with drug dosing, potential errors, and practice-specific events.
Preparing for Change
After gathering support and input from each clinical service line involved in infusion therapy, we created an infusion pump drug library that established dosing limits for each drug and a clinical protocol for verifying overrides or dosing outside the limits. When dosing outside facility-established dosing parameters, the infusion pump alerts the clinician, then prompts a change and confirmation step. We chose the smart pump in part for its ability to allow “soft stop” overrides — that is, allowing the clinician to appropriately override the dosing parameters to achieve therapeutic effect or to exceed the limit in an emergency — but also because it allowed for customization and a more uniform, reliable approach to safety.
Before converting to new infusion pumps, we successfully piloted the software program with three select drugs in the ICU and labor/delivery departments. Recognizing that there would be a significant investment of time to extrapolate our initial findings throughout the rest of the organization, we decided to move forward with the full conversion.
During this evaluation period, we realized a one-size-fits-all drug library would not benefit our institution, nor would it help us to improve patient safety to the standards we had set for ourselves. We wanted to be able to gather all relevant data from these pumps, and be able to apply key findings to daily practice within the hospital.
The terms of our success rested on the teamwork among nursing, pharmacy, and prescribing clinicians to determine the drugs most often used — and most likely to cause a potential error or concern. We spent more than 6 months in small working groups to evaluate our needs and decide the best course of action for using these drugs safely in the future. The teams included representatives from critical care, oncology, obstetrics, medical/surgical, and pediatrics, all of whom had a professional stake in how medications are administered. We reviewed drug dosing guidelines for each of the 48 infusion-delivered medications included in our library, and based on the evidence-based literature, established our own protocols for each. These guidelines, approved by the interdisciplinary team, took into consideration circumstances that require dosing parameters to be altered, such as with pediatric patients.
As we charted new territory with the smart pump, we also took a more critical look at our own staff’s practices. In response, we developed a protocol that required two nurses at the bedside to verify overrides and a progress note reflecting the override and ordering physician. Doctors could still prescribe as necessary for the patient, but the addition of a second nurse at the bedside provided much needed verification for entering a high or low dose of the prescribed infusion.
Results
In January 2006 we implemented 275 pumps in the designated departments and by April 2006 collected data from a random sample of 36 pumps (12 each from critical care, medical/surgical and obstetrics). With the help of our vendor, we evaluated our first 3 months of usage to create a full picture of events including dose alerts (attempts to program outside the limits), overrides (doses outside the limits that were accepted), and potential errors (doses that were rejected).
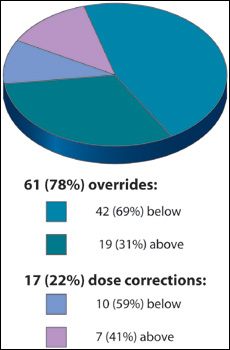
Of 2,536 total doses, 78 dose alerts occurred. Of these 78 alerts, the majority (78%) were overridden; that is, the dose was accepted. This represented a trend of dosing titratable drugs both above and below the published dosing limits to achieve patient therapeutic effect. Sixty-nine percent of our overridden alerts were below the limit and associated with titrating to patient effect or weaning off the drug. For example, phenylephrine registered 30 total alerts, but 29 of those were actually below the set limit. Titratable drugs delivered below the limit included phenylephrine, milrinone, amiodarone, diltiazem, nicardipine, and fentanyl. Three drugs revealed trends of programming above the limit: vasopressin, oxytocin, and magnesium sulfate. Oxytocin registered 17 total alerts, all of which were above the limit (See Table 1).
|
Medical
For each of these drugs, the doses were corrected to “within range” only four times, indicating that it was the protocol and drug library limits that may have needed adjustment, rather than clinician or prescriber action. In fact, this prompted a reassessment of protocol and dosing parameters for the drug phenylephrine.
 Phenylephrine represented an interesting challenge to us because of its beneficial hemodynamic properties at a variety of dosage ranges, but relatively little evidence-based literature to support its high dose prescribing — leading to our original, lower dosing parameters. Based on the dose alert data, however, we re-evaluated these parameters, eventually modifying our policy to incorporate an intensivist review and approval to pharmacy for any high therapy. We will also modify the pump setting to include the newly accepted dosing range.
Phenylephrine represented an interesting challenge to us because of its beneficial hemodynamic properties at a variety of dosage ranges, but relatively little evidence-based literature to support its high dose prescribing — leading to our original, lower dosing parameters. Based on the dose alert data, however, we re-evaluated these parameters, eventually modifying our policy to incorporate an intensivist review and approval to pharmacy for any high therapy. We will also modify the pump setting to include the newly accepted dosing range.
Further analysis of the dose alert data also revealed 2 of the 78 alerts represented averted dosing events that could have lead to a significant patient event:
- Phenylephrine was programmed at 1,747 mcg/min, which was 385.3% above the limit (dose range 40-360 mcg/min), but after receiving the alert, was reprogrammed at 80 mcg/min.

- Oxytocin was programmed at 166.5 milliU/min, which was 732.5% above the limit (dose range 0.25-20 milliU/min), but after receiving the alert, was reprogrammed at 8 milliU/min.
These potential medication events were averted by the software and dosing parameters we put in place — proving the efficacy of the dose-limiting technology. With clinical analysis and interpretation of the dosing alerts, we were able to classify the different types of overrides and corrections so that we could identify medication events and adjust practices for best outcomes.
Discussion
The data we retrieved from the dose-tracking software validated the hospital’s decision to convert to the new dose limiting technology. We were able to better understand our institution’s needs and help ensure no dosing event would result in an adverse patient event. We could assess whether the dosing overrides were necessary and appropriately verified, and present the data to our colleagues for legitimate discussion and action, instead of solely relying on anecdotal reports.
As data became available regarding significant “below limit” overrides, we made adjustments to the library settings, further enhancing the effectiveness of our dose alert system. The process of utilizing the pumps and new protocols causes us to continually review our practices and make necessary changes, including our re-evaluation of phenylephrine protocol.
We were pleased to find only 2 of the 2,536 doses represented averted medication events. This was fewer than we expected, indicating a very low incidence of manual programming error associated with this infusion device. Our results gave us confidence in the smart-pump technology, our dosing protocols, and the dosing practices of our clinicians.
We were also encouraged to discover a concrete reduction in infusion-related medication events at Lutheran Medical after our implementation of the smart-pump technology and evidence-based protocols; from a total of seven events in 2005 to only three in 2006 — a 57% decrease.
Through the conversion process, we also learned that the success of any large technology conversion rests not only on the teamwork and commitment of internal staff, but in the commitment of the vendor to provide comprehensive services and programs, including round-the-clock education and technical support, customized training materials, continuing education, and data analysis support. With staff buy-in from across the hospital, we were pleased that all departments experienced the benefits of this technology specific to their practice area.
Conclusion
Smart pumps help to ensure patient safety and encourage better outcomes — but simply booting up a program or recording data is just the beginning. By customizing our drug library system to best fit our institution’s needs, and by using our vendor’s thorough expert data analysis and clinical interpretation service, we were able to utilize this technology for continual improvement. In just a few months’ time, the smart-pump technology significantly reduced our infusion-related medication events — and just as importantly, the data analysis helped us identify key areas for further improvement. The results of our commitment to combine technology implementation with critical process improvement initiatives were measurable patient safety improvements today and a system designed to expand with the future.
Rosanne Raso is the senior vice president for nursing services at Lutheran Medical Center in Brooklyn, New York. She previously held two chief nursing officer roles in community hospitals in New York and New Jersey and is most happy when establishing warm and collaborative patient care environments where nurses are supported and appreciated for the extraordinary work they do. Raso graduated from an associate degree program in Staten Island, New York, in 1970 and began her nursing career as a critical care nurse. She earned her master’s degree in 1985 and has enjoyed many clinical and administrative leadership roles since then. Raso serves on the editorial board of Nursing Management and NY/NJ Nursing Spectrum. She is a past president of the New York Organization of Nurse Executives, the New York City Chapter AACN, and the Greater New York Chapter of the New York Organization of Nurse Executives. Raso may be contacted at rraso@lmcmc.com.
Joan Velletri has served as director of nursing education at Lutheran Medical for the past 5 years. She is responsible for selecting and supervising clinical instructors and providing for the clinical training and education needs of all nursing staff. She also collaborates with clinical leadership to maintain and improve standards of nursing practice. Velletri has more than 15 years of experience as a staff development instructor at Lutheran Medical Center, and currently serves on advisory boards for various New York-area nursing programs, including Kingsborough Community College, New York University, and Touro. She received her BSN and MSN from the Hunter College Bellevue School of Nursing, New York, New York.
Steven DiCrescento is the director of pharmacy at Lutheran Medical Center, where he is responsible for support of all clinical, distributive, technological, regulatory, and patient safety aspects, including staff education, medication management, and Joint Commission standards compliance. He previously served at the NYU Medical Center for 4 years, and at Bellevue Hospital Center for 13 years. He is also a member of the American College of Clinical Pharmacy, New York State Council Health-System Pharmacists, American Pharmacists Association, and is a New York State-licensed pharmacist.
References
AHA, Health Research & Educational Trust, & ISMP. (2002). Pathways for medication safety: Leading a strategic planning effort.
Institute of Medicine. (2006). Preventing medication errors. P. Aspden, J. Wolcott, J. L. Bootman, L. R. Cronenwett, (Eds.). Washington, DC: National Academy Press.
Tourville, J. (2003). Automation and error reduction: How technology is helping Children’s Medical Center of Dallas reach zero-tolerance. US Pharmacist, 28(6), 80-86.
