Informed Consent: Comprehension is the Key
May / June 2006
Informed Consent:
Comprehension is the Key
The challenge hospitals face is that many patients do not understand the fundamental information regarding their treatment plans.
Most hospital processes focus on trying to prevent costly medical errors after a patient begins treatment. However, there is a process that starts before patient treatment that can have an even bigger impact on patient safety and quality of care. The informed consent process ideally serves as a way to educate patients about their conditions; the benefits, risks, and potential complications of a procedure; treatment alternatives; and risks associated with not performing the procedure. At present, all of this information is packaged typically as a generic form coupled with a conversation between patient and physician.
The challenge hospitals face is that many patients do not understand the fundamental information regarding their treatment plans. Even though a patient may sign a consent form stating that he or she understands what has been communicated, studies show that even after agreeing to or receiving care, 18% to 45% of patients are unable to recall the major risks associated with their surgeries (Graham, 2003; Saw, et al., 1994; Cassileth, et al., 1980); many cannot answer basic questions about the services or procedures they agreed to receive (Wadey & Frank, 1997; Berlanger, et al., 1980; Lavelle-Jones, et al., 1993); 44% do not know the exact nature of their operation (Byrne, et al., 1988); and most (60%) do not understand or read (60% to 69%) the information contained in the generic hospital informed consent forms (Parker, 2000). Obviously, the lack of fully comprehended informed consent presents significant patient safety and quality of care risks and can also lead to increased medical malpractice risk for hospitals and for physicians.
As forward-thinking hospitals realize this liability and consider ways to address it, emerging technology offers a cost-effective and easily-implemented solution that not only decreases the risk of adverse malpractice judgments, settlements, and legal costs but also improves the quality of care by enhancing the communication between the physician and the patient.
Quality and Safety Organizations Lead the Push
The 2000 publication of the Institute of Medicine (IOM) report, To Err Is Human: Building a Safer Health System, captured the attention of the public and policymakers relative to the patient safety risks associated with medical care. In response to these findings, the Agency for Healthcare Research and Quality commissioned its own report to evaluate various evidence-based, best safety practices. Published in 2001, Making Health Care Safer: A Critical Analysis of Patient Safety Practicesreviewed 79 patient safety practices and focused special attention on 11 practices deserving of widespread implementation. One of these practices was: “Asking that patients recall and restate what they have been told during the informed consent process.” This was an effort to ensure that patients not only read and heard the informed consent, but more importantly, understood the informed consent.
Further, the National Quality Forum (NQF), an organization chartered to develop and implement a national strategy for healthcare quality measurement and reporting, released a report, Safe Practices for Better Healthcare,that endorsed a set of national, voluntary consensus standards for 30 healthcare practices aimed at improving patient safety throughout the healthcare system. This strategy, called Safe Practice 10, states that all healthcare professionals should ask patients to repeat or “teach back” what they have been told by their provider during the informed consent discussion.
In December 2003, the NQF initiated a project to accelerate widespread adoption of Safe Practice 10. That effort resulted in the September 2005 publication of an implementation report, Improving Patient Safety Through Informed Consent for Patients with Limited Health Literacy, and a user’s guide, Implementing a National Voluntary Consensus Standard for Informed Consent.The following real-life examples from the user’s guide demonstrate the need for Safe Practice 10:
- A Spanish-speaking woman walked out of the hospital just prior to surgery when it was finally communicated clearly to her that tubal ligation was a permanent sterilization technique, not a temporary method of birth control.
- The planned anesthesia for a patient was incompatible with Coumadin, but the patient did not report use of Coumadin until “teach back” was used at a late stage of the pre-operative process, allowing providers to avoid a potentially fatal interaction.
- Four months after starting to use “teach back,” one hospital department, which frequently saw 100 patients per day, dropped the surgical cancellation delay rate from 8% to 0.8%, resulting in a savings of $56 per minute for the resources that previously had been wasted by cancelled or delayed surgeries.
Technology Helps Meet Emerging Guidelines
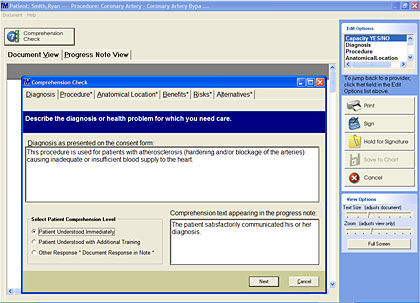
As NQF’s efforts to spur adoption of its Safe Practices for Better Healthcarecontinue to gain ground, hospitals are encouraged to address the inconsistencies and inadequacies of the traditional informed consent process with an eye toward achieving fully comprehended informed consent on a consistent basis. Many hospitals are finding that an automated informed consent application is the best way to meet these and other emerging guidelines (Figure 1).
![]()
Automated Informed Consent
To date, hospitals have taken a manual, non-standardized approach to informed consent, relying on generic fill-in-the-blank forms that are created by the provider. Information collected through this approach may vary and can be inconsistent since inclusion depends on an individual’s judgment to determine a patient’s understanding of the procedure.
Designed to standardize clinical communication, an automated informed consent application offers providers access to a comprehensive library of detailed, procedure-specific forms that include explanations of risks, benefits, and alternatives; prognosis if the patient elects not to undergo treatment; and other relevant topics. All materials are written in a style that is patient-friendly and easy to understand—not in complicated medical or legal jargon. The automated tool frees a provider to focus on communicating critical aspects of a contemplated procedure and not on preparing a fill-in-the-blank form. This opportunity for enhanced communication, coupled with the availability of easy-to-understand documentation, is critical to a patient being able to comprehend and “teach back” basic information about his or her procedure.
The ultimate benefit of an automated informed consent process is its ability to help increase patient safety and improve quality of care. As they relate to NQF objectives, hospitals have found that automated informed consent applications give providers a tool that can be used as a means to ensure patients fully understand the various aspects of a planned procedure.
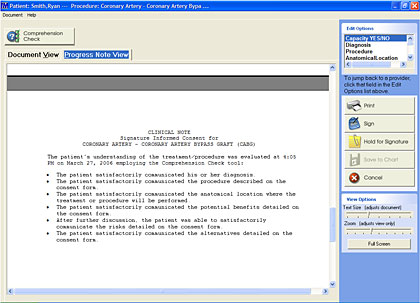
For example, the NQF’s informed consent user’s guide reminds providers that simply asking questions such as “Do you understand?” or “Do you have any questions?” may not result in patients communicating whether or not they really understand aspects of a proposed treatment. Some automated informed consent applications offer providers a series of specific questions designed to quantify patient understanding—not simply the ability of a patient to pass a “quiz.” Those automated tools also provide the ability to easily document that verbal communication with the patient, confirming that he or she understands key components of a procedure. Lastly, an automated informed consent solution will automatically apply a note to the patient’s chart, or electronic medical record, stating that he or she gave fully-comprehended informed consent (Figure 2).
![]()
An automated informed consent application also contributes to ensuring that hospitals are in compliance with other established regulations and requirements. For Medicare reimbursement, Centers for Medicare & Medicaid Services (CMS) guidelines state that if patients do not understand what they are consenting to, then it is not informed consent. The Joint Commission on Accreditation of Healthcare Organizations (JCAHO) standard for informed consent notes that the goal of informed consent is mutual understanding between the patient and physician. And The Leapfrog Group Hospital Quality and Safety Survey assesses hospitals across the country according to the 30 practices in NQF’s Safe Practices for Better Healthcare,including Safe Practice 10 on informed consent.
Hospitals and physicians are presently being challenged to improve the informed consent process. The government, the American Medical Association (AMA) and accreditation organizations are demanding that the process meet all of the guidelines for truly informing the patient about contemplated procedures and tests. Implementing a process to ensure fully-comprehended informed consent is a means to accomplish this previously daunting task. As a result, patients will be better informed and more compliant as they will not only be adequately educated but will also comprehend what is going to be done. The future of medicine will depend on hospitals and doctors making these needed changes and leveraging new technology that improves the quality of healthcare.
|
Neil Baum is a practicing urologist and associate clinical professor of urology at Tulane Medical School and Louisiana State University Medical School. He delivered a paper entitled Automating Informed Consent: Are You Overlooking a Safety Opportunity? at the 2006 HIMSS Conference and may be contacted at NeilB89@aol.com.
References
Agency for Healthcare Research and Quality (AHRQ). (2001). Making health care safer: A critical analysis of patient safety practices. www.ahrq.gov/CLINIC/PTSAFETY/index.html
Bergler, J. H., Pennington, A. C., Metcalfe, M., & Freis, E. D. (1980). Informed consent: How much does the patient understand? Clinical Pharmacology and Therapeutics, 27(4), 435-440.
Byrne, D. J., Napier, A., & Cuschieri, A. (1988). How informed is signed consent? British Medical Journal (Clinical Research Edition), 296(6625):839-840.
Cassileth, B. R., Zupkis, R. V., Sutton-Smith, K., & March, V. (1980). Informed consent Why are its goals so imperfectly realized? New England Journal of Medicine, 302(16), 896-900.
Graham, P. (2003). Type of consent does not influence patient recall of serious potential radiation toxicity of adjuvant breast radiotherapy. Australasian Radiology, 47(4), 416.
Institute of Medicine. (2000). To err is human: Building a safer health system.L. T. Kohn, J. M. Corrigan, & M. S. Donaldson (Eds.). Washington, DC: National Academy Press.
Lavelle-Jones, C., Byrne, D. J., Rice, P., & Cuschieri, A. (1993). Factors affecting quality of informed consent. British Medical Journal, 306(6882), 885-890.
National Quality Forum. (2005). Implementing a national voluntary consensus standard for informed consent – A user’s guide for healthcare professionals. Washington DC: Author. www.qualityforum.org/publications.html.
National Quality Forum. (2005). Improving patient safety through informed consent for patients with limited health literacy An implementation report. Washington DC: Author. www.qualityforum.org/publications.html.
Parker, R. (2000). Health literacy: A challenge for American patients and their health care providers. Health Promotion International, 15(4), 277-283.
Saw, K. C., Wood , A. M., Murphy, K., Parry, J. R., & Hartfall, W. G. (1994). Informed consent: An evaluation of patients’ understanding and opinion (with respect to the operation of transurethral resection of prostate). Journal of the Royal Society of Medicine, 87(3), 143-4.
Wadey, V., Frank, C. (1997). The effectiveness of patient verbalization on informed consent. Canadian Journal of Surgery, 40(2), 124-128.
