Heart Safe or Heart Break: What Every Clinician Needs to Know About AEDs
September / October 2006
Heart Safe or Heart Break: What Every Clinician Needs to Know About AEDs
![]()
Cardiac arrest is a leading cause of death in the United States. The American Heart Association estimates that this year 250,000 Americans will die of sudden cardiac arrest before reaching a hospital. That’s 680 deaths each day; one death every 2 minutes.
Sudden cardiac arrest usually occurs without warning and can strike people of all ages, even physically fit, apparently healthy adults and children. Eighty percent of cardiac arrests are caused by ventricular fibrillation (VF), an abnormal heart rhythm that prevents the heart from pumping blood effectively. The only treatment for VF is immediate electrical heart defibrillation.
When cardiac arrest occurs outside of a hospital, defibrillation is administered through an automated electronic defibrillator (AED). An AED is a lifesaving medical device that evaluates the cardiac status of a person who is suspected of suffering from cardiac arrest. It contains a microprocessor that analyzes the heart’s rhythm for abnormalities and, if necessary, guides the user through the process of administering a defibrillation shock. This shock causes all of the muscles in the heart to contract with the goal of jolting it out of the fatal rhythm and restoring a more natural, healthier heartbeat.
AEDs are easy to operate and can be used with minimal training. Once turned on, an AED instructs the user to place the pads and electrodes on the victim’s chest then issues a series of verbal and visual instructions to guide the user through the defibrillation process.
Time is the most important factor in a sudden cardiac emergency. The odds of survival decrease 7% to 10% for every minute without immediate cardiopulmonary resuscitation (CPR) and defibrillation. After 10 minutes without defibrillation, few resuscitation attempts are successful (AHA). Administering CPR while waiting for a defibrillator to arrive can increase a victim’s chance of survival because it keeps the blood flowing to the heart and brain. CPR is also important immediately following defibrillation until the heart is able to pump effectively. The American Heart Association (AHA) estimates that at least 20,000 lives can be saved annually by delivering defibrillation with AEDs (National Conference of State Legislatures).
Recalls, Failures, and Other Dangers
The good news is that ready-access to AEDs saves the lives of thousands of cardiac arrest victims every year. The bad news is that many AEDs — more than 100,000 — have been recalled in the last 3 years. More than 30,000 AEDs have been recalled in 2006 alone. This trend in defibrillator recalls was noticed by Risk And Safety Management Alert System (RASMAS) analysts at Mitretek Systems, who compiled a list of 103,553 AEDs that have been recalled since the beginning in Sept. 2003 (See Table 1). Full details of these recalls, including device serial numbers, are available http://rasmas.mitretek.org/AED.html.
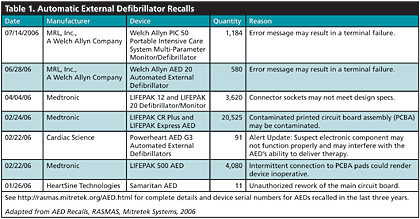
![]()
These recalls are due to a variety of causes — faulty circuit boards, incorrect battery fuses, failure to deliver an electric shock, and potential battery cell ruptures. The problem is so widespread that these recalls involve at least seven different manufacturers.
Mitretek’s observations were validated by a 10-year study of AEDs published in The Journal of The American Medicine Association in August 2006. This study, conducted between 1996 and 2005, showed that one in five AEDs was recalled due to potential malfunction. The study states that in this 10-year period, the U.S. Food and Drug Administration (FDA) issued 52 advisories involving AEDs or critical AED accessories affecting 385,922 devices (Jignesh, et al., 2006).
Clinical Implications
As a result of these recalls, some manufacturers, such as Access CardioSystems, have gone out of business and no longer provide service, repair, or technical support for their AEDs. They have also stopped selling replacements for disposable parts such as battery packs and electrode sets.
Hospitals, AED program administrators, and responders need to be aware of how frequently AEDs are recalled, and they need to make sure that the devices located in the public areas of their facilities are checked, tested, and maintained on a regular basis. They also need to know what to do when their devices have been recalled and how to update or repair them. In the event that an AED cannot be serviced or replacement parts are unavailable, the AED must be replaced. Without proper maintenance or upkeep, AEDs can be a patient safety hazard and potential liability risk.
The Benefits Outweigh the Risks and Recalls
Although the failure rate of AEDs is startling, the medical community has made great strides in understanding resuscitation and cardiac arrest and has published myriad national and international studies showing the overwhelming benefits of having publicly accessible AEDs. Early research established the connection between survival rates and positive quality of life after out-of-hospital defibrillation. It also proved that reducing the interval between a victim’s collapse and the administration of defibrillation has the greatest impact on survival rates following cardiac arrest.
In 1991, the AHA published “Improving Survival from Sudden Cardiac Arrest: The Chain of Survival Concept.” This paper recommended that all communities adopt the principle of early defibrillation and that everyone qualified to perform CPR should also be trained to use AEDs. In the mid 1990s, the AHA launched a public health initiative to promote early CPR and early use of AEDs.
A 1999 study, “Comparison of Naive Sixth-Grade Children with Trained Professionals in the Use of an Automated External Defibrillator,” by the AHA bolstered early support for the public placement of AEDs (Gundry, et al., 1999). This videotaped simulation of a cardiac arrest compared the speed in which trained emergency responders administered defibrillation with an AED to the speed in which untrained sixth-grade children were able to offer defibrillation to a mannequin. The results found a surprisingly small difference in response times between the two groups — 69 to 111 seconds for the untrained children versus 50 to 87 seconds for trained emergency professionals. The findings suggested that widespread use of AEDs would require only modest training.
These recommendations were strengthened by additional studies that showed positive outcomes and healthy lifestyles in victims who received defibrillation with an AED in an out-of-hospital cardiac arrest. A Scottish study published in the British Medical Journal in June 1996, showed that 40% of 1,476 patients resuscitated from out-of-hospital cardiac arrest were discharged without major neurological damage and that nearly 70% of patients discharged after cardiac arrest were alive 4 years after the event.
Similarly, a 2003 study, “Long-Term Outcomes of Out-of-Hospital Cardiac Arrest After Successful Early Defibrillation,” showed that the long-term survival of patients who experienced rapid out-of-hospital defibrillation following cardiac arrest was “similar to that among age-, sex-, and disease-matched patients who did not have out-of-hospital cardiac arrest.” The study went on to say that the quality of life of most of the survivors was “similar to that of the general population. (Bunch, et al., 2003)”
AEDs Save Lives
Today, AEDs are widely recognized as the greatest lifesaving advancement for victims of cardiac arrest since the discovery of CPR. Accessible, well-maintained AEDs that are free from recalls are the key to survival for victims of cardiac arrest. Studies have shown that four events are generally associated with positive outcomes following an out-of-hospital cardiac arrest:
- The victim’s cardiac arrest was witnessed by someone who called 911 or other appropriate medical personnel.

- Someone immediately began to resuscitate the victim using CPR.

- Someone arrived with a working AED and administered defibrillation within 3 to 5 minutes after collapse.

- Emergency support personnel arrived quickly and offered advanced life support within 8 minutes of cardiac arrest
AED Legislation: A Commitment to the Community; a Commitment to Saving Lives
In April of 1997, Arizona enacted the first broad public access law pertaining to AEDs. As of May 2001, all 50 states had enacted defibrillator laws or adopted regulations concerning their use. Many of these laws allocated state funds to purchase AEDs and create AED programs, directed the placement of AEDs in public facilities, and limited the liability of lay rescuers.
Widespread deployment of AEDs began in October 2000 when President Clinton signed the Cardiac Arrest Survival Act (CASA, Public Law 106-505). This law directed the placement of AEDs in Federal buildings and also provides liability protection for those who render emergency treatment with an AED. Another important provision was that CASA superseded state laws by extending “Good Samaritan” law protections to AED users in all states. CASA triggered wide acceptance of AEDs as lifesaving devices and paved the way for facilities such as hospitals, nursing homes, airports, and country clubs to begin purchasing these devices under the public-access defibrillation (PAD) program.
Home Use of AEDs — A New Healthcare Challenge
In September of 2004, public access to AEDs changed radically when the FDA approved the Philips HeartStart Home Defibrillator for over-the-counter (OTC) sale without a prescription. The device is now available for purchase from Amazon.com for $1,100.
To receive clearance for OTC sales from the FDA, Philips had to demonstrate that its Home Defibrillator model could be used safely and effectively without medical supervision. Philips conducted usability tests proving that even untrained individuals could follow the device’s instructions and carry out a defibrillation shock safely.
By FDA regulation, AEDs are tracked devices. This means that manufacturers must have mechanisms in place that allow them to identify the location of AEDs involved in a recall. The FDA states that these tracking programs are effective and that “more than 95 percent of the AEDs affected by Class I recalls in 2005 were returned to the manufacturers or taken out of service. (FDA, 2006)”
When AEDs are purchased over the counter, they are shipped with enrollment forms and warranty cards to help manufacturers contact owners in a recall emergency. If purchasers fail to fill out and return the cards, it makes it difficult for manufacturers to inform them about device problems and recalls. Tracking and communications problems can be compounded when AEDs are purchased second- or third-hand.
AED buyers, patient education programs, and physicians recommending in-home defibrillators need to ensure that their patients understand the importance of returning reply cards and registering their AEDs so that they can be contacted by manufacturers in the event of a recall. They must also be educated about the necessity of rigid AED maintenance. Many of the best practices outlined for PAD program operators can be adopted for home-use AEDs.
Creation and Proliferation of PAD Programs
The goal of an AED or PAD program is to increase the rate of survival of people who have a sudden cardiac arrest. The AHA believes that by strategically placing AEDs throughout communities and training non-healthcare professionals to use them, more than 50,000 lives can be saved annually. But to save lives, AEDs must be in working order, properly maintained, free from recalls, and accessible.
Successful PAD programs require attention to planning, maintenance, and training. Important program success factors involve interaction with trained emergency personnel, the development of detailed training plans, conducting routine maintenance including checking for recalled devices, and the incorporation of quality improvement measures.
Best Practices for Staffing PAD Programs
A successful PAD program must have strong support personnel. Perhaps the most important is the onsite coordinator who oversees the day-to-day operations of the program. Ideally, the coordinator should have experience with AEDs and resuscitation programs. The onsite coordinator should act as an advocate for the AED program and should communicate the program’s mission, goals, and decisions with the AED’s owners, the businesses policy makers, emergency responders, employees, and the public. The coordinator should:
- develop detailed, site-specific emergency response plans,

- develop an onsite responder team,

- coordinate training and retraining for all designated responders,

- develop a thorough written AED maintenance plan and testing schedule, and

- act as the liaison for offsite program staff such as physicians and emergency medical personnel.
Understanding state and local laws surrounding the use of AEDs is important for the program coordinator because registering a PAD program with emergency personnel is sometimes required by law. Some states also require that emergency services personnel collect heart rhythm and device data that are captured by AEDs during each use. This information is typically obtained from a card within the device, transferred from the device via modem or manually captured by filling out an AED incident form.
The location of AEDs is also frequently dictated by state or local laws. Exact placement may be determined by the program coordinator with recommendations from emergency personnel or a physician. The placement should be less than a 2-minute walk from places where people congregate or locations that have been identified as high-risk or high-traffic areas. The locations of all AEDs should be communicated to emergency personnel so that they can direct 911 callers to the nearest AED.
Regardless of whether it is required by law, developing a partnership with local emergency personnel is an important step in establishing a successful PAD program. A good understanding of the procedures involved in transferring care from your defibrillation program into the hands of emergency technicians can save precious minutes for victims of cardiac arrest.
It is also important to establish a strong relationship with area hospitals because hospitals are frequently the best source for information about the operation of AEDs and the development of PAD programs. This support typically includes consultations or advice concerning
- device purchase,

- medical support and supervision,

- training and education, and

- competency assessments.
Most hospitals offer state-certified CPR/AED courses and refresher classes for hospital staff and emergency personnel, as well as outreach and training programs for the public. Additionally, some hospitals have services that inform them about medical product and device recalls, including AEDs. If your program is associated with a hospital, ask the risk management department if they have such a program and if they will forward all AED-related recall information to your program coordinator. You may also be able to provide them with device serial numbers and ask them to keep you informed in the event that any of your program’s devices are recalled.
Another essential PAD program member is a physician. The FDA classifies AEDs as Class 3 medical devices and, with the exception of some home defibrillators, they can only be purchased with a prescription. The prescription requirement is intended to ensure that AEDs are used in organized programs with appropriate medical planning and supervision and that the devices can be located during a recall.
A PAD program physician writes AED prescriptions and provides medical oversight for the program’s implementation. This frequently includes helping to develop sound emergency procedures and emergency action plans. In many cases, the physician will also provide post-use reviews as well as ongoing support and quality improvement.
The Importance of AED Maintenance
AEDs are sophisticated devices that require on-site maintenance. Most AEDs have internal component testing functions that perform self-maintenance, but — like smoke alarms or fire extinguishers — AED devices cannot be just hung on a wall and left there. Each manufacturer has its own recommended maintenance and testing schedule. Some require testing every day. A written maintenance plan should be developed and followed.
An onsite person should be appointed to perform daily maintenance and site inspections of all AEDs to ensure that they are in top form in case of an emergency. AED maintenance should include:
- Verifying that all AEDs are in their proper locations and that they have not been damaged or tampered with.

- Monitoring external status lights indicating that the AED is in proper working order.

- Knowing the expiration dates of AED batteries and other disposable parts and replacing them as necessary.

- Inspecting AEDs for damage and replenishing supplies after each use.
The increasing availability of these sophisticated devices to the general public and the critical role they play in emergency care makes their maintenance extremely important,” says Bill Klein, director of alert quality control at Mitretek Systems National Risk And Safety Management Alert System (RASMAS) Center. “Every organization that makes these devices available to staff and visitors should make sure a maintenance schedule is in place and that their policy and procedures reflect a means for dealing with manufacturer recalls.”
Maintaining, Replacing, Retraining, and Other Expenses
Money needs to be budgeted for the operational expenses of an AED program. Personnel expenses include program support, onsite maintenance, and responder training. The AHA recommends that all responders receive a refresher course every 6 months and complete retraining every 2 years. Money must also be allocated for AED maintenance, supplies, and replacement. This is especially important as AEDs age.
The 5-Year Mark
In the past 10 years, there has been a 10-fold increase in the number of AEDs purchased. In 1996, only 20,000 AEDs were available in public locations. By 2005, this number had ballooned to 200,000. As we approach the 5-year mark since most AEDs have been installed, it is imperative to check AED warranties and batteries. Mitretek’s review of nine popular AED models found that the majority of AED batteries expire after 5 years on the shelf. The FDA reports that dead batteries are the most common reason for AED device failure and that many defibrillators and batteries remain in use beyond the service life intended by the manufacturer.
Environmental factors such as exposure to extreme temperature and usage also impact battery life. AEDs that reside in outdoor stadiums and cars have a shorter lifespan than those that are not exposed to cold or heat. Each use of an AED also drains life from the battery. A trained technician should evaluate the AED after each use. Special attention should be paid to the recommendations of the manufacturers as some recommend replacing the battery after each use.
The Communication Gap: Contacting AED Owners
The proliferation of AEDs has made it more difficult for manufacturers to contact people who have purchased AEDs. Manufacturers must know how to communicate with AED owners and operators if there are problems with a device or if a recall has been issued. Owners should also know how to contact the manufacturer if they encounter problems with their units. To increase communication with manufacturers and improve device effectiveness, program coordinators need to:
- Ensure that the maintenance schedule recommended by the manufacturer is followed.

- Repair and replace AEDs and their related components as recommended by the manufacturer.

- Remain vigilant about recalls and knows how to contact the manufacturer if necessary.

- Post the name of the office responsible for the AEDs and their maintenance on or nearby the unit.

- Return all AED warranty cards promptly. (Best practice dictates that the card should state the name and address of the office responsible for AED. Tying the recall information to an office rather than a particular user ensures that recall information will be delivered, even if there has been staff turnover.)
Conclusion
Proactive campaigns and legislation have led to the proliferation of AEDs in the last decade, and these devices have saved thousands of lives. The extraordinarily high recall rate of AEDs in recent years is dismaying. However, since the chance of surviving sudden cardiac arrest without defibrillation is minimal, the benefits of making AEDs available to the public far outweigh the risks. By following established best practices for maintenance and training, clinicians, PAD program administrators, and home AED users can ensure that their AEDs are in top form and able to save lives in an emergency.
Laury Sendek is a new media strategist for Mitretek Systems in Falls Church, Virginia. She has over 20 years of experience in the communications and information technology fields. Prior to working with Mitretek, she was employed by Northrop Grumman Information Technology. She can be contacted at laury.sendek@mitretek.org.
References
American Heart Association. Cardiac arrest. http://www.americanheart.org/presenter.jhtml?identifier=4481
Bunch, T. J., White, R. D., Bernard J. Gersh, B. J., Meverden, R. A., Hodge, D. O., Ballman,, K. V., et al. (2003). Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation. New England Journal of Medicine, 348, 2626-2633. http://content.nejm.org/cgi/content/short/348/26/2626
Cobbe, S. M., Dalziel, K., Ford, I., & Marsden, A. K. (1996). Survival of 1476 patients initially resuscitated from out of hospital cardiac arrest. British Medical Journal, 312, 1633-1637. http://bmj.bmjjournals.com/cgi/content/full/312/7047/1633?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=1&author1=Cobbe&andorexacttitle=and&andorexacttitleabs=and&andorexactfulltext=and&searchid=1&FIRSTINDEX=0&sortspec=relevance&resourcetype=HWCIT
FDA Statement. (2006, August 10). Journal of American Medical Association article on recalls and safety alerts affecting automated external defibrillators. http://www.fda.gov/bbs/topics/NEWS/2006/NEW01429.html
Gundry, J. W., Comess, K. A., DeRook, F. A., Jorgenson, D., & Bardy, G. H. (1999). Comparison of naive sixth-grade children with trained professionals in the use of an automated external defibrillator. Circulation, 100, 1703-1707. http://circ.ahajournals.org/cgi/content/full/100/16/1703
National Conference of State Legislatures, (2006, June). State laws on heart attacks, cardiac arrest & defibrillators. http://www.ncsl.org/programs/health/aed.htm
Shah, J. S.,& Maisel, W. H. (2006). Recalls and safety alerts affecting automated external defibrillators. The Journal of the American Medical Association, 296, 655-660. http://jama.ama-assn.org/cgi/content/full/296/6/655
