Do Not Let “Depo-” Medications Be a Depot for Mistakes
By the Institute for Safe Medication Practices
Problem:
Today, several longstanding medications are available on the market with names that begin with the prefix “Depo-,” meaning they are administered via a depot injection that deposits the drug into localized tissue from which it is gradually absorbed by surrounding tissue. These injections, typically subcutaneous, intramuscular, or intra-articular, allow the active compound to be released consistently over a longer period of time. Many of these medications with the prefix “Depo-” have been on the market for 30 to 50 years, some even longer. Misadministration of these medications by the intravenous (IV) route has been consistently reported throughout the years, as has confusing one “Depo-” medication with another. More recently, mix-ups between different strengths and volumes of containers of a “Depo-” drug have occurred. The “Depo-” medications most often involved in these wrong route, wrong drug, or wrong strength/volume errors include:
DEPO-PROVERA, DEPO-PROVERA Contraceptive Injection, and DEPO-SUBQ PROVERA 104 (medroxyPROGESTERone acetate): an intramuscular or subcutaneous progestin used as a contraceptive, or to treat endometriosis or endometrial carcinoma.
DEPO-MEDROL (methylPREDNISolone acetate): an anti-inflammatory or immunosuppressive corticosteroid given via intramuscular or intra-articular injection.
DEPO-TESTOSTERONE (testosterone cypionate): an intramuscular androgen used to treat male hypogonadism.
Examples of error reports submitted to the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) and/or the ISMP National Medication Errors Reporting Program (MERP) are provided below.
Depo-Provera vs. Depo-Medrol
In early 2015, FAERS received a report involving a 44-year-old man with shoulder pain (non-impingement) who received an intra-articular injection of Depo-Provera instead of Depo-Medrol. A box of Depo-Provera had been stored inadvertently in the bi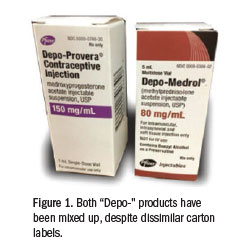 n where Depo-Medrol was usually kept in a medication cabinet. Other than size, the medication cartons do not look similar, but both drug names start with “Depo-…” and appear in the same black font (Figure 1). The physician performing the shoulder injection reached into the bin and removed a box of the correct medication, Depo-Medrol, and read the label and concentration. He set that carton aside, and a moment later, he inadvertently reached back into the bin and removed another box, but this one contained Depo-Provera. Believing he had previously confirmed the drug and concentration, the physician withdrew the desired amount of an opaque white liquid similar in appearance to Depo-Medrol (Figure 2). The physician injected about three-quarters of the 1 mL volume he had withdrawn into the syringe (about 112 mg of Depo-Provera). A medical assistant discovered the error while cleaning up after the patient had left the office. Within three days of injection, the patient experienced a lack of libido and had erectile dysfunction, which required prolonged use of testosterone and CIALIS (tadalafil). Since making the error, the physician learned that others have made the same error, mixing up Depo-Medrol and Depo-Provera.
n where Depo-Medrol was usually kept in a medication cabinet. Other than size, the medication cartons do not look similar, but both drug names start with “Depo-…” and appear in the same black font (Figure 1). The physician performing the shoulder injection reached into the bin and removed a box of the correct medication, Depo-Medrol, and read the label and concentration. He set that carton aside, and a moment later, he inadvertently reached back into the bin and removed another box, but this one contained Depo-Provera. Believing he had previously confirmed the drug and concentration, the physician withdrew the desired amount of an opaque white liquid similar in appearance to Depo-Medrol (Figure 2). The physician injected about three-quarters of the 1 mL volume he had withdrawn into the syringe (about 112 mg of Depo-Provera). A medical assistant discovered the error while cleaning up after the patient had left the office. Within three days of injection, the patient experienced a lack of libido and had erectile dysfunction, which required prolonged use of testosterone and CIALIS (tadalafil). Since making the error, the physician learned that others have made the same error, mixing up Depo-Medrol and Depo-Provera.
Depo-Medrol vs. Depo-Provera
Several years ago, ISMP received a report of a similar mix-up, again leading to patient harm, but in this case, the patient was supposed to receive Depo-Provera but received Depo-Medrol in error. A 19-year-old woman went to a clinic to receive an injection of Depo-Provera for contraception, which was to be repeated every 12 weeks. After providing a negative pregnancy test, the young woman was mistakenly given an intramuscular injection of Depo-Medrol. The lot number of the vial of medication was recorded in her medical record. The woman returned in 12 weeks and reported a positive home pregnancy test. An ultrasound confirmed the pregnancy, with an estimated date of conception about 3 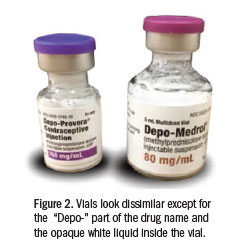 ½ weeks after her first injection. The error was uncovered when the documented lot number was found to be associated with Depo-Medrol.
½ weeks after her first injection. The error was uncovered when the documented lot number was found to be associated with Depo-Medrol.
In this case, Depo-Medrol and Depo-Provera had previously been stored in separate cabinets. However, a few days before the event, the medication cabinets had been consolidated, and the medications were stored alphabetically in bins. The stock in the consolidated cabinet had been labeled with Depo-Provera and Depo-Medrol, which were stored next to each other. Working with only a verbal order for the drug, the clinic staff had accidentally selected a vial of Depo-Medrol instead of the intended Depo-Provera.
