Dangerous Wrong-Route Errors With Tranexamic Acid—A Major Cause for Concern
By the Institute for Safe Medication Practices
Problem:
Earlier this year, ISMP was notified about two cases of accidental intraspinal injection of tranexamic acid, occurring in two states. Unfortunately, the report was sent anonymously, and we were unable to learn additional details about the events, including the outcome of each patient.* In previously reported cases, mix-ups mostly occurred with vials/ampules of tranexamic acid and bupivacaine or ropivacaine when selecting products prior to regional anesthesia. In the United States, all three drugs can be found packaged in vials with the same blue plastic cap (Figures 1 and 2).
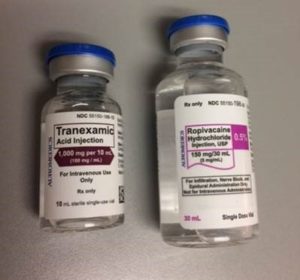

Figure 1: While the label colors and vial sizes are different, the caps on ropvacaine and tranexamic acid vials (left), each from AuroMedics, are both blue and could lead staff to select a vial based on cap color without reading the label, especially if the vials are stored upright with only the caps showing (right), not the labels.
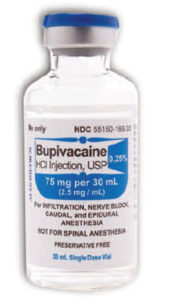
Figure 2: AuroMedics’ bupivacaine vial also has a blue cap.
While label colors may be different, when the vials are stored upright, in close proximity to one another, and in a bin, drawer, or below eye level, only the caps may be visible. To make matters worse, these drugs are often stored in areas where bar code scanning may not have been implemented or is not routinely utilized (e.g., perioperative areas, labor and delivery, emergency department). So, mix-ups are less likely to be detected.
Tranexamic acid is an antifibrinolytic that is used in a variety of hemorrhagic conditions to control bleeding, including postpartum hemorrhage. It works by preventing the breakdown of fibrin, thus promoting clotting. When given intraspinally instead of a local anesthetic, tranexamic acid is a potent neurotoxin with a mortality rate of about 50% and is almost always harmful to the patient. Survivors of intraspinal tranexamic acid injection often experience seizures, permanent neurological injury, and paraplegia (Palanisamy & Kinsella, 2019). Unfortunately, the literature is replete with reports of serious medication errors due to mix-ups between tranexamic acid and bupivacaine or ropivacaine during regional anesthesia.
In 2015, we wrote about a 68-year-old woman undergoing a total knee replacement who received tranexamic acid intrathecally instead of the intended bupivacaine and morphine. The surgeon planned to irrigate the open knee with tranexamic acid to minimize blood loss, and the anesthesiologist intended to administer bupivacaine and morphine intrathecally for pain control. Pharmacy dispensed 10 mL vials of both the tranexamic acid (1 g or 100 mg/mL) and bupivacaine (2.5 mg/mL) to the surgical suite for staff to prepare in syringes just prior to administration. The vials were similar in size. Despite label dissimilarities, the anesthesiologist picked up a 10 mL vial of tranexamic acid, believing it was bupivacaine.
The anesthesiologist later administered the tranexamic acid intraspinally. The bar code on the medication had not been scanned before preparation and administration. The patient immediately developed myoclonus of her lower extremities and seizures. She was intubated and placed on a ventilator while receiving a neuromuscular blocker. She required treatment in the ICU, and after 23 days of hospitalization, the patient was finally discharged to a skilled nursing facility for physical rehabilitation.
A recent review article identified 21 cases of spinal tranexamic acid administration (Patel, Robertson, & McConachie, 2019). Other case reports are listed in the references provided in our 2015 newsletter article.
Readers may be aware of a new neuraxial connector that is becoming available, known as NRFit®. These connectors may be helpful in preventing wrong-route errors but won’t prevent all mix-ups. Since regional anesthesia is generally administered as a one-time event in an operating or delivery room, the local anesthetic is usually drawn into a syringe by anesthesia personnel and injected via the intraspinal catheter (with an NRFit connector, if used). If a vial mix-up occurs and tranexamic acid is drawn into an NRFit syringe that is compatible with the NRFit catheter, it’s still possible for the wrong drug to be injected. Of course, bar code scanning and pharmacy preparation of prefilled syringes or infusions would help to alleviate these errors.
Last month, Exela Pharma Sciences received approval to manufacture a premixed bag of tranexamic acid in a sodium chloride solution for injection as 1 g/100 mL (10 mg/mL), as opposed to vials of 1 g per 10 mL (100 mg/mL) (www.ismp.org/ext/251). The company is preparing to launch this product within the next quarter. Although approval was granted specifically for patients with hemophilia to reduce the risk of hemorrhage during and following tooth extraction, the premixed product could find use with other forms of bleeding. A 100 mL bag would greatly reduce the potential for mix-ups with bupivacaine or ropivacaine vials; however, use of the infusion product and dosing information for any condition other than the approved indication is not provided in the product labeling. Also, since loading doses may be required prior to infusion, vials/ampules may still be needed (or a smart infusion pump loading dose feature could be used that automatically switches to a continuous infusion once the loading dose has been delivered). *
*ISMP discourages individuals from submitting reports anonymously. When reports are sent anonymously, it becomes impossible to learn additional information that may be key to understanding what took place and the root causes of an event. ISMP always holds report information in strict confidence, as do the U.S. Food and Drug Administration (FDA) and manufacturers, with whom we communicate. We do not share reporter information with the FDA or manufacturers without your permission. Also, as noted in the error reporting section of the ISMP website, upon request, reports can be sent to ISMP as a federally recognized Patient Safety Organization (PSO), which affords further protection from discovery during a plaintiff’s lawsuit.

Safe Practice Recommendations
Avoid storing these drug vials in an upright position to prevent reliance on identifying the drug by viewing only the vial caps. Store them in a way that always makes their labels visible. Minimize look-alike vials or ampules by purchasing these products from different manufacturers, and separate or sequester tranexamic acid in storage locations to provide an additional layer of safety against errors. We also recommend using an auxiliary label to indicate the intended route of administration on all tranexamic acid container labels.
Originally published in the May 23, 2019 issue of Acute Care ISMP Medication Safety Alert!
References
Palanisamy, A., & Kinsella, S. M. (2019). Spinal tranexamic acid – a new killer in town. Anaesthesia, April 15, 2019 [Epub ahead of print]. Retrieved from www.ismp.org/ext/263
Patel, S., Robertson, B., & McConachie, I. (2019). Catastrophic drug errors involving tranexamic acid administered during spinal anesthesia. Anaesthesia, April 15, 2019 [Epub ahead of print]. Retrieved from www.ismp.org/ext/264
