An Interdisciplinary Approach to Safer Blood Transfusion
March / April 2008
![]()
An Interdisciplinary Approach to Safer Blood Transfusion
![]()
For many healthcare professionals, the concept of hazardous blood transfusion is defined by concerns about transfusion-mediated disease. Thus it is understandable that the focus during the last three decades has been on improving blood safety (safety of the fluid) to the extent that we have seen a significant decline in transfusion-transmitted disease. So it is appropriate now to focus attention on improving the overall safety of blood transfusion, a complex and multi-step process that has long been recognized as a significant patient-safety issue (Dzik, 2005).
Between 1976 and 1985, the FDA reported 265 transfusion-related deaths (Sazama, 1990). Over half were caused by acute hemolytic reactions following transfusion of ABO-incompatible products (Sazama, 1990). Linden’s report of 1998 transfusion statistics further illustrated the frequency of transfusion errors and their outcomes (Linden, 1999). These included mistakes during: (1) blood collection, (2) wristband concordance verification, (3) blood storage, and (4) specimen labeling. These patient-safety issues will soon have financial implications for hospitals when new Medicare and Medicaid provisions are implemented in 2008 that no longer permit national healthcare systems to absorb hospital costs associated with transfusion errors (Federal Register, 2007).
St. Luke’s Episcopal Hospital is a not-for-profit 627 bed tertiary care teaching facility in the Texas Medical Center in Houston, Texas. As home of the Texas Heart Institute, the hospital specializes in cardiovascular medicine. The hospital transfuses more than 3,000 blood products per month. Maintaining an adequate blood supply for patients is facilitated by an in-house blood donor program that supplies approximately 70% of the blood needs for the hospital. For the last 5 years, St. Luke’s has been engaged in a multi-pronged and multidisciplinary effort for enhancing the safety of blood transfusion. Implementation of new technologies and process improvements has addressed the complete transfusion cycle including the accuracy of blood product labeling and tracking, the transportation of blood products to the correct patient, the accurate identification of the patient at the point of transfusion, and the appropriate utilization of blood products.
Improved Product Labeling and Tracking ISBT-128
The ABC Codabar system, developed in the 1970s, used linear barcode technology to encode numeric information. Unfortunately, the quantity of unique barcode numbers was limited, and further use of the Codabar system risked the possibility of repetition of blood product identification, creating the dangerous potential for serologically distinct blood products bearing the same label. In 1994, the International Society of Blood Transfusion (ISBT) revised the standards for information distribution with regard to transfusion and transplantation (ICCBBA, 2006) and issued the ISBT-128 specifications with the recommendation for conversion by 1998. ISBT-128 technology enhances the information capacity of a simple barcode. Each product receives a label with a 13-character identifier, which designates the collection facility, collection year, and donation sequence number. A two-dimensional data matrix code then stores the information for use in future machine-readable and electronic data storage technologies. In addition, ISBT-128 standardizes the labeling format to provide unique product codes and data identifiers for each barcode for blood product identification.
Although blood banks and transfusion medicine specialists have recognized that ISBT-128 improves the safety of labeling and tracking blood products, implementation of a new, nationwide system for blood collection has not been universally embraced. Many large institutions have been resistant to the logistical and financial burdens of change. However, the 2006 update to the FDA’s Blood labeling Industry Guidance standard required all blood and blood component container labels to include barcodes for the unique facility identifier, donor number, donor ABO/Rh, and product code (U.S. Food and Drug Administration, 2006) This would require facilities to move toward barcode labels that would allow scanning for blood products prior to administration similar to that required for medications. More recently the American Association of Blood Banks (AABB) has moved to require ISBT-128 labeling by all facilities by May 2008. (AABB, 2005).
In 2002, St. Luke’s Episcopal Health System became the first non-military hospital to adopt the ISBT-128 technology (Southwick, 2003). Because the St. Luke’s Blood Donor Program provides the majority of the hospital’s transfused products and does not ship products to other hospitals, St. Luke’s was able to move forward and set the new standards for transfusion safety in the Gulf Coast region. Citing the elimination “of the possibility of error” as the prompt toward the system conversion, St. Luke’s became a leader in the trend toward change, and labeled all collected blood products with ISBT-128 labels. Early adoption of the improved labeling system was made possible when the Blood Donor Center installed a new electronic information system that had the ability to collect, reprocess, track donor infectious disease testing, label, and ship ISBT blood products. In 2004 the hospital completed installation of software allowing the Transfusion Service Laboratory to modify and re-label ISBT-128 products, making St. Luke’s Episcopal Hospital the first US hospital to issue ISBT-128 labeled products for transfusion. In addition, the software included expert logic allowing an electronic crossmatch process to check for ABO compatibility, a further enhancement to patient-safety.
Process Improvement for Blood Transport
In late 2006, St. Luke’s adopted Lean process improvement as its standard operating procedure. The most distinctive project undertaken during the early months of the hospital’s Lean campaign didn’t address the normal efficiency and cost opportunities arising from the world-famous Toyota Production System that the hospital hoped to emulate. The Lean-improvement project associated with the transport of blood between the hospital’s transfusion service laboratory, cardiovascular operating rooms, and cardiovascular recovery room was focused exclusively on patient safety.
Because of the nature of cardiovascular surgery, patients often need blood transfusions. At St. Luke’s units of blood for a given patient are placed in coolers with ice packs and transported to the operating rooms. The blood units in the coolers have a “shelf life” of 8 hours before unused units must be returned to the transfusion service laboratory. Transportation involves passing the coolers through a number of individuals between the transfusion service laboratory, cardiovascular operating rooms, and cardiovascular recovery room. After a near-miss involving the transportation of the wrong blood products to a patient, a multidisciplinary team of laboratory technologists, patient care assistants, nurses, and physicians was formed to examine and redesign the process of transporting blood products. The team first examined the current state of blood transport for the operating rooms and found certain deficiencies. There were points during transport system where the blood in coolers was not adequately tracked, with no one having accountability for the cooler, where it was, or who was responsible for it. There was also a lack of metrics and controls for recording this information. Not only was this recognized as a potential patient safety issue, the current state resulted in incidents of wasted blood products when coolers were not returned to the transfusion service laboratory within the 8-hour time frame.
The team employed various Lean methodologies including standardization, job instruction training, metrics for tracking waste, visual management, 5S, and Kaizen (Womack et al., 1996). The major opportunity for process-improvement came with the recognition that the old documentation attached to a cooler was inadequate, consisting of only the patient’s information, return time and who issued the blood from the transfusion service laboratory. Nothing else was recorded for the duration of the cooler’s journey. The documentation form was modified to create a tracking method, so that every time a hand-off of the cooler occurred, there would be a “from” signature for the person who was giving it to someone else, and a “to” signature of the recipient. Each deliverer and recipient in the transport chain now has to initial and write his or her badge number on the new documentation attached to the coolers. At certain points along the way, the coolers’ contents are checked, to make sure they contain what the label specifies. Now, with more accountability from employees who transport blood and an auditable paper trail, there’s far less chance of coolers ending up where they shouldn’t be or blood products being wasted when coolers are not returned to the transfusion service laboratory in a timely manner. The new process created better communication among the staff that transports blood. Similar to a chain of custody, people are now more attentive in making sure the blood gets to its appropriate destination.
Another important improvement came with the elimination of one of the blood coolers’ stops on the way to the operating rooms. Just prior to arriving in the operating rooms, patients and coolers were kept in a pre-operative holding area. Such a layover for the coolers had no functional value and only provided an extra chance for errors to occur. Now coolers bypass the holding area altogether and go directly to the operating room. In the operating room itself, a simple piece of visual management helped to secure the coolers where they’re needed most. Formerly, they were positioned randomly in the room, set down in no particular spot. Now, a colored-tape footprint on the floor marks the spot where the cooler has to be placed. With visual management, people recognize where the cooler should be located and are more apt to ask questions when it’s not in its specified location.
As of early May 2007, the Blood Cooler Transport Initiative achieved several important metrics including 97% reduction in late returned coolers, 100% reduction of platelet wastage due to inappropriate placement in coolers (platelets must be maintained at room temperature), 100% reduction of blood wastage from the late return of coolers, and 97% reduction of calls from transfusion service laboratory inquiring about late coolers. But the most important achievement was the significant reduction in the occurrence of negative variances associated with blood transfusion. These negative variances may be thought of as near misses that occur when improperly transported blood is brought to the bedside of the wrong patient. Although other safeguards in place may prevent incompatible blood from being transfused into the patient (the worst case scenario), these variances represent potential patient safety hazards that should be eliminated.
Electronic Patient ID Verification for Blood Administration
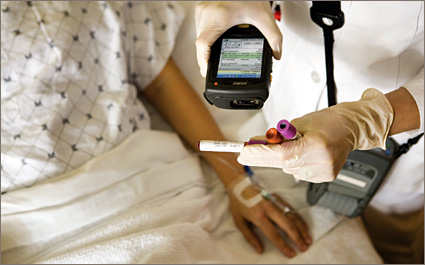
The most recent safety initiative completed by St. Luke’s has been the implementation of an electronic transfusion verification system throughout the hospital, which enabled scanning of products and patients just prior to blood administration. With the scan of the patient’s armband barcode and the barcode label on the blood product, a handheld wireless device (the system was compatible with the wireless network already in place) protects patients by confirming patient and blood product identification at the bedside. The system not only crosschecks electronic orders to reduce potential for ABO incompatible transfusion, but also documents transfusion data in the electronic patient record.
With thousands of transfusions performed monthly, implementation of electronic verification required a multidisciplinary approach involving nurses, physicians, laboratory technologists, and information management specialists. The group designed a roadmap for server/wireless installation, software configuration, and interface design. Classroom and web-based instruction as well as one-on-one tutorials provided staff training. A team of nursing education and laboratory support staff assisted with pilot studies, roll-out schedules, system implementation, and training of more than 800 users. Despite the challenges, the end result is a safer, more efficient, and integrated transfusion process and improved documentation of every transfusion in the electronic patient record. Transfusion messages sent from the wireless devices document the actual transfusion date/time in both the transfusion service laboratory information system and the hospital information system, providing better audit capability.
The initial step in blood transfusion involves the acquisition of a patient specimen for type and crossmatch so that appropriately matched blood products are prepared for transfusion. Accurate patient identification before specimen collection is paramount. In 2008 St. Luke’s will launch a specimen collection verification system for documentation and tracking of blood specimens. Using the same barcode scan technology described above, this system documents collection data, follows specimens, prints facility-specific labels, and verifies safety parameters, including order and patient identification. Labels are then scanned whenever patient information and test results are entered thus avoiding patient identification errors and closing the loop on patient identification from specimen collection to blood administration. Moreover, the system advances the safety and efficiency of the entire specimen collection process and will be used for the collection of all patient specimens submitted for laboratory analysis.
Improving Blood Utilization Enhances Patient Safety
According to the 2005 Nationwide Blood Collection and Utilization Survey Report, over 14 million units of blood were transfused in 2004 at mean cost of $201.07 per unit (AABB survey, 2005). While blood centers and hospitals coordinate to meet current demand, the margin between supply and demand is ever diminishing. Collaborative efforts to conserve blood must be implemented to ensure a cost-effective and sustainable blood supply for patients.
Last year St. Luke’s implemented an electronic blood product order entry system, allowing physicians to order specific blood products and list a specific reason for transfusion. Orders for specific products allowed utilization of expert rules to insure patient blood product special needs are met. Appropriate issue of leukoreduced or irradiated products when indicated improves patient safety. Provision of the reason for transfusion allows the transfusion service laboratory to audit transfusion requests and ensure proper use and conservation of blood products. The hospital’s information management team also developed interfaces between the order entry system and the hospital information system, displaying blood order status and eliminating the need for caregivers to telephone the transfusion service laboratory to determine if blood products were available for pick-up. These interfaces also provided the foundation for implementing a transfusion verification system.
The most significant initiative that will impact blood utilization for the hospital is its integrated blood conservation program, begun in late 2007. There is increasing awareness among healthcare professionals that a large number of transfusions are given unnecessarily (Eaton, 2004). An integrated blood conservation program is a proactive, system-wide approach to standardize and utilize modalities and protocols that promote safe and appropriate blood usage and ultimately reduce the number of blood transfusions. Conservation is achieved with a comprehensive, multidisciplinary process designed to promote a universal standard of care in transfusion therapy. Subject matter expertise is essential to create a successful program in a relatively short time period. To this end, St. Luke’s has partnered with a consulting group to facilitate program implementation. A dedicated manager — a clinician trained in blood conservation processes — oversees the program. The program manager develops standardized policies and procedures, provides education to clinical and administrative staff, and assures consistent standard of care. Education is achieved through evidenced-based data collection and analysis using the consultant’s proprietary software. A successful integrated blood conservation program can preserve blood supply resources, reduce the risk of blood-borne diseases and transfusion-mediated immune suppression, promote faster healing, and accommodate patient’s transfusion preferences.
In summary, by investing in new technologies, leveraging information management capabilities, and embracing proven process improvement methods, St. Luke’s Episcopal Hospital is at the forefront of providing safe, medically appropriate blood transfusions to its patients.
|
Mark LaRocco is assistant vice president and patient safety officer at St Luke’s Episcopal Hospital in Houston, Texas. He has more than 30 years experience as a hospital laboratory specialist. LaRocco has been with St. Luke’s since 1991, first as director of microbiology then as administrative director of pathology before taking on his current roles.ÝLaRocco may be contacted at mlarocco@sleh.com.
Kathy Brient is also at St. Luke’s Episcopal Hospital, where she is the manager of laboratory information systems (LIS). She has been a member of the pathology department at St. Luke’s for more than 30 years, first as a medical technologist specializing in blood banking then as LIS manager. She has extensive experience in transfusion service laboratory medicine and blood administration technologies as well as laboratory information management systems.
References
AABB. (2005). ISBT-128 Implementation Plan, Association Bulletin #05-12. Bethesda Md.: American Association of Blood Banks.
AABB Survey. (2005). The 2005 nationwide blood collection and utilization survey report. Available at: http://www.aabb.org/apps/docs/05nbcusrpt.pdf.
Dzik, W. (2005). Technology for enhanced transfusion safety. American Society for Hematology.
Eaton, L. (2004). Chief medical officer warns that many blood transfusions are unnecessary. BMJ, 329, 308.
ICCBBA.(2006). Introduction to ISBT-128, 3rd edition. P. Ashford (Ed.). York, PA: Author. Available at: http://iccbba.org/ISBT128introbooklet.pdf
Linden, JV. (1999). Errors in Transfusion Medicine. Archives of Pathology and Laboratory Medicine, 123, 563-565.
Medicare program: changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Fed Regist 2007;72:47379-47428.Ý
Sazama, K. (1990). Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion, 30, 583-590.
Southwick, K. (2003, October). Waiting game nearly over for ISBT-128. CAP Today, October, page 1. Northfield, Ill.: College of American Pathologists.
U.S. Food and Drug Administration. Guidance for industry-recognition and use of a standard for uniform blood and blood component container labels. Available at: http://www.fda.gov/cber/gdlns/unilabbld.htm
Womack, J. and Jones, D. (1996). Lean thinking. New York, N.Y.: Simon and Schuster.
