A Better Alternative for Combating Opioid Misuse Without Restricting Drug Access for Pain Patients
By Terrence O’Neill
Since 1999, deaths due to drug overdose have topped 1 million, according to the Centers for Disease Control and Prevention’s (CDC) National Center for Health Statistics.
A number of drugs contributed to these fatalities, but the death rates began to rise significantly when opioid medications started to be marketed and prescribed for more common aches and pains. The journey to addiction and death often begins with an injury or legitimate medical need leading to the use of or access to painkillers. Then as the addiction strengthens its hold, tolerances increase, more doses are required, savings are spent, and individuals turn to street drugs that are not only cheaper, but also sometimes contain fentanyl, a powerful and deadly synthetic opioid.
On February 10, the CDC released a draft of new proposed guidelines for prescribing opioid painkillers. The draft removes the 2016 recommended ceilings on prescription doses for chronic pain patients and instead encourages doctors to exercise their best judgment. Even though the previous dosing ceilings were recommendations, they led to unintended consequences: States codified them, and physicians concerned with criminal or civil penalties misapplied the rigid standards by tapering patients too quickly or even refusing to provide treatment.
Payer regulations further complicate pain treatment. A 2019 survey conducted by the American Board of Pain Medicine found that 92% of pain specialists reported that they were required to submit a prior authorization for nonopioid pain care that delayed treatment and required extra staff to manage the administrative burden.
Prescription drug monitoring programs unable to stem opioid misuse
To date, efforts have focused on stemming the supply of opioids available for misuse by instituting prescription drug monitoring programs (PDMP) that identify doctor shoppers (patients who visit multiple doctors to obtain opioid prescriptions) and pill mills (providers who write opioid prescriptions without a legitimate medical purpose). PDMPs were rolled out state by state and required a coordinated, sustained effort to implement and secure funding. Studies regarding the effectiveness of PDMPs have been mixed, but we are all better off when barriers are raised against the unchecked flow of opioids.
However, people who misuse painkillers have preferred sources for obtaining opioids, and the data consistently identifies those sources. According to the Substance Abuse and Mental Health Services Administration’s (SAMHSA) National Survey of Drug Use and Health, people age 12 or older who misused pain relievers in the past year overwhelmingly obtained their opioids from one doctor or from a friend or relative—opioid supply sources PDMPs are not designed to track.
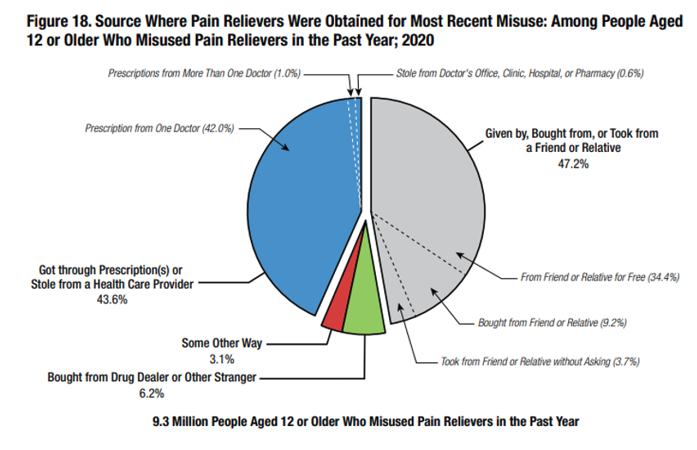
Patient adherence left unmonitored
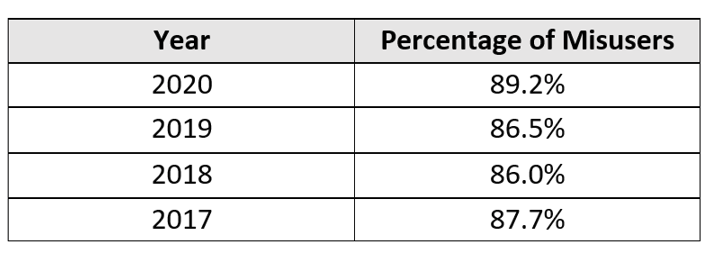
The latest SAMHSA data regarding the preferred sources of misused opioids (i.e., the percentage of users who received their opioids from a single doctor or from a friend/relative) over a four-year period is illustrated below. Despite this trend, we have achieved little focus, coordination, or progress in monitoring patient adherence for those prescribed opioids.
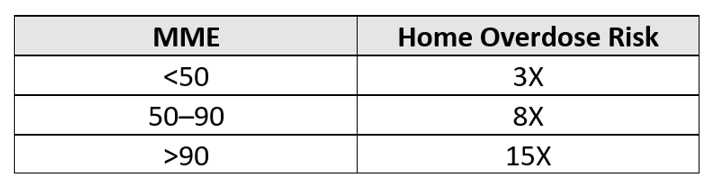
Additionally, opioids present risks to more than the patient: The mere presence of opioids also creates significant dangers for everyone in the home. An observational study published in the British Medical Journal (BMJ) in 2020, “Opioid Prescribing Patterns Among Medical Providers in the United States, 2003-17,” compared homes with opioids versus homes without opioids. Depending on the morphine milligram equivalent (MME) of the opioid, the likelihood of an overdose in homes with opioids increased significantly as illustrated in the matrix below. These increased risks are particularly concerning in cases when the patient is being victimized, unaware that someone they trust is stealing their medication. In fairness to the patient, it is difficult to keep track of tablets dispensed in amber vials.
As the “opioid epidemic” raged on, many of the organizations involved in supplying opioids (i.e., manufacturers, distributors, and dispensers) found themselves defendants in multibillion-dollar lawsuits for providing quantities of opioids to communities that far exceeded a reasonable level of expected demand. While it is important to hold those accountable who prioritized profits over lives, it is unclear how the settlement payouts will create positive change to address the underlying issues. Just as the PDMP rollout required a nationwide strategy, funding, and sustained effort, and just as the FDA created a multiyear plan to implement the Drug Supply Chain Security Act (DSCSA) to protect consumers from exposure to harmful drugs, we must exercise similar determination and coordination to start addressing the other causes and challenges that have led to overdoses and deaths.
How to ensure the right patient is taking the right dose at the right time
The fact that opioids are still predominantly dispensed in amber vials is astonishing. Reasonable adherence solutions exist that can not only link specific drugs to specific patients, but also help ensure that the right patient is taking the right dose at the right time. These solutions leverage common technology and materials, can be personalized depending on patient needs, and allow providers to develop evidence-based data. When integrated with sound pain or addiction treatment practices such as pill counts and urine drug screening, these solutions can provide an ROI well beyond medication compliance.
Barriers to treatment exist for many patients and many reasons. For example, in communities with limited or no local treatment options, patients must travel to receive care, which may involve long distances or public transit challenges. Adherence monitoring solutions create transparency that can be leveraged along with telehealth solutions, enabling providers to reach beyond the walls of a practice and reduce patient access inequities. These solutions can also boost patient engagement, reinforcing the benefit-risk analysis of the medication and treatment goals. Cloud-based systems can track patient medication adherence and, during random pill counts, enable providers to instantly verify that patients are presenting medications dispensed specifically to them, even during telehealth calls.
According to Frank James, medical director of outpatient services at Mind Springs Health, “These tools create evidence-based information and memorialize best prescribing practices across the continuum of care. Additionally, they promote data sharing across one’s practice and with allied health professionals.”
For patients, pragmatic solutions create a real-time connection to the provider and make it easier for them to understand their level of adherence. A smartphone application can inform patients of the time since their last dose and how many tablets they should have left, which is particularly useful for patients managing multiple medications. This level of visibility can decrease the risk of unintended overdose and make it easier to identify when a patient is being victimized.
When longitudinal patient adherence data is shared with other stakeholders involved in the patient’s care, we also have an opportunity to reduce costs. Predetermined, measurable outcomes can be aligned with alternative payment models to help demonstrate high-quality, cost-efficient care.
But as documented in the National Institutes of Health’s National Pain Strategy in 2016, there is a need to “create a payment and delivery environment that facilitates coordinated care across the continuum of pain and throughout the lifespan in order to conform to the biopsychological model and provide value, as defined by the outcomes of care.” Thus, to support fully integrated adherence monitoring programs, we need financial flows that reimburse providers and pharmacies who support and leverage enhanced adherence monitoring.
The benefits mentioned in this article have focused on pain and addiction treatment but can be applied to any medications that are prone to misuse or to treatments that have significant patient adherence challenges. Even high-cost specialty drugs that involve burdensome administration or risk-based contracting can benefit from increased visibility regarding patient adherence.
Manufacturing and treatment consolidation creates opportunities for success
Many believe that healthcare is too large or too complex to make these types of meaningful change. The PDMP implementation, and the ongoing progress of the DSCSA rollout, demonstrate otherwise and showcase the power of sustained motivation and coordination.
Consider these statistics relative to opioid distribution and treatment. In 2019, The Washington Post published an article, “76 Billion Opioid Pills: Newly Released Federal Data Unmasks the Epidemic.” In it, a review of a Drug Enforcement Administration database indicated that from 2006 to 2012, just three companies manufactured 88% of opioids and six companies distributed/dispensed 75% of them. The aforementioned BMJ observational study also determined that the top 1% of providers prescribing opioids accounted for a significant portion of overall opioid distribution, as illustrated in the matrix below.
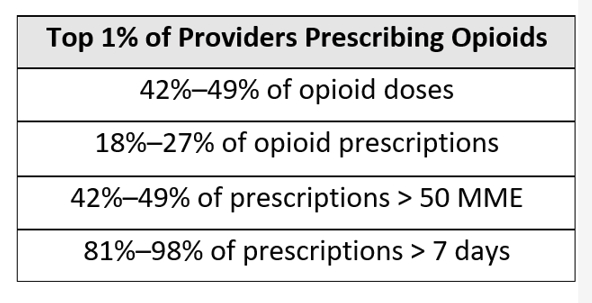
With this type of consolidation on both the supply and demand side of opioids, we could make rapid progress if those few influencers participated in well-planned patient adherence programs—ones that focus on developing evidence-based, personalized solutions and that leverage existing materials and technology.
Although more than 1 million people have died from drug overdoses, their journeys often began with access to legitimately prescribed opioids. We can address the opioid dilemma by reducing overdose risks and improving pain/addiction treatment outcomes; we just need to muster the will and commitment to advance patient opioid adherence.
Terrence O’Neill is director of business development at Covectra, a leader in track-and-trace solutions.
