Medication Reconciliation in Daily Rounds in the NICU
September/October 2011
Medication Reconciliation in Daily Rounds in the NICU
There is a major thrust for patient safety nationwide. With an estimated 1.5 million preventable adverse drug events (ADEs) occurring annually in the United States, there is still a need for better error prevention systems (Institute of Medicine, 2007). Medication errors are the most frequent type of errors made in hospital settings and are common among pediatric settings, especially in the neonatal intensive care unit (NICU) (Suresh et al., 2004; Kaushal et al., 2001). Errors have been shown to be eight times more likely in the NICU compared to adult patient settings due to a variety of factors, including a neonate’s growing body size, changing developmental systems, weight-based dosages, off-label drug usage, and the inability of the provider to communicate with the patient (Kaushel et al., 2001; Kozer et al., 2002; Conroy et al., 2000; Raju et al., 1989).
One method of preventing common medication errors is the practice of medication reconciliation. The Joint Commission (2006) defines medication reconciliation as consisting of three steps: verification, clarification, and reconciliation of the patient’s medication list. By resolving discrepancies, medication reconciliation ensures an accurate accounting of the patient’s medication list to prevent common errors such as wrong medications, omissions, duplications, dosing errors, or drug interactions (Hall, 2010). Since neonates are especially vulnerable to such errors, it is important to practice medication reconciliation in the NICU not only during transitions of care but also on a routine basis throughout an infant’s stay.
Past studies tested the idea of medication reconciliations only during transfers of care such as admissions and discharges (The Joint Commission, 2006; Hall, 2010; Coffee et al., 2009; Greenwald et al., 2010; Mills & McGuffie, 2010; Unroe et al., 2010). These transition points frequently are associated with changes in medication regimens, making such periods vulnerable to medication errors. In one study within a surgical ICU, nearly all medication errors in discharge orders were eliminated after 6 months of medication reconciliation implementation (Pronovost et al., 2003). Another study demonstrated how medication reconciliation during admissions in an emergency department reduced the prescribing error rate from 3.3 errors per patient to 0.04 errors (Mills & McGuffie, 2010).
Despite growing evidence of its importance, the medication reconciliation process is still poorly practiced in most units and has not been studied during a patient’s non-transfer inpatient stay. Studying medication reconciliation during these times has been made more practical with the use of electronic health records (EHR) that include computerized physician-order entry systems. Electronic medical ordering systems allow clinicians by the bedside to have each patient’s current and complete list of medications. With the daily changes made for neonates in the NICU, medication reconciliations could be useful on a more frequent basis. It is also important for physicians to be involved in medication reconciliation during this process as they are ultimately responsible for the patient’s medication management (American Medical Association, 2007). However most medication reconciliations are initiated and led by pharmacists in the unit (Mills & McGuffie, 2010; Murphy et al., 2009). Due to the frequent lack of dedicated clinical pharmacists in NICUs, this is not always possible. Therefore we sought to implement physician-led medication reconciliation practice during a patient’s tay within a hospital unit.
The aim of this study is to evaluate a physician-led medication accountability practice during daily bed-rounds in a NICU and to understand essential features of this process and the factors that are important in successful implementation.
Methods
Setting
The study took place in a 75-bed NICU in a 450-bed university-based Children’s Hospital. The unit is made up of individual patient rooms (separate pods with three walls with one to three beds) and multi-bed rooms (open bays fitting two to seven beds). It is divided into three wings: East, Central, and West. The East and Central wings are composed primarily of multi-bed pods, and the West wing is composed of isolated patient rooms.
The newborn care team includes attending physicians, fellows, residents, house physicians, physician assistants, neonatal nurse practitioners, staff nurses, specialists, social workers, and dietitians. Three medical teams (Team A, B, C) exist in the unit, and each team is led by an attending physician. In conjunction with a supervising neonatologist and a neonatal fellow, frontline care on Teams A and B is provided by pediatric residents with a team size of 15 to 17 patients. Frontline care on Team C is provided by nurse practitioners, physician assistants, and house physicians with a team size of 18 to 20 patients.
Mobile computers are available for each medical team, which provide clinicians with access to the electronic ordering system available throughout the unit. At the time of the study, Sunrise Clinical Manager® was the acute care electronic health record used within the hospital.
Staff Education
After observing the prescribing process, our research team found that medication reconciliation was not routinely practiced in the unit. Staff education was initiated among the NICU staff about the medication reconciliation process through clinical conferences and focus groups. A team member presented medication reconciliations at a clinical conference attended by attending physicians and frontline clinicians in the unit. In this conference, the potential for medication reconciliation in reducing medical errors was emphasized and the process was explained. All questions and concerns from the staff were addressed at this time. In addition, each medical team was educated about the nature of medication reconciliation before morning rounds. We reviewed the steps involved in the medication reconciliation process with prescribers.
Medication Accountability System
In the context of our study, medication reconciliation (Med Rec) is defined as a read-back of the current and active medication list for a patient. This list was read out-loud by the prescriber from the mobile computer assigned to the team and usually took no more than one minute.
Using the Plan, Do, Study, Act (PDSA) performance model, the Med Rec practice was tested during morning bed-rounds. Two observers (ML, SS) followed the three medical teams in the NICU during bed-rounds for a period of 1 month (July 2010). Baseline data among all three teams were collected before Med Rec was implemented. After educating the staff on the process of Med Rec, it was put into practice and monitored by one of two observers for a period of 2 months (September and October 2010).
Outcome Metrics
Two independent observers timed the length of Med Rec and bedside rounds, the frequency of Med Rec, and any order changes and clarifications attributable to Med Recs. A change is defined as any addition or discontinuation of medication(s); a change in the dose, route, or frequency of a medication(s), or the replacement of one medication with another medication. A clarification is defined as addressing any team member’s question or concern prompted by the Med Rec. All changes and clarifications could be seen from the mobile laptop where the prescriber accessed the hospital electronic health records.
The average length of bed-rounding time per patient and the time of medication reconciliations per patient were calculated for each medical team. The percentage of bed-rounding time dedicated to medication reconciliations was also calculated.
The following two variable factors were analyzed: 1) the bed arrangement and 2) the members of the medical teams. The environment took into account the two types of patient rooms: isolated rooms and multi-bed pods. Team A rounded on patients that were primarily in the open pods (west side) and Team B rounded on patients primarily in the isolated rooms (east side). Team C was not included in this analysis because they rounded in all areas of the NICU. Another factor was the different composition among all three medical teams. Both Teams A and B were resident driven, and Team C was nurse practitioner and physician assistant driven. All teams were led by an attending physician.
Data Analysis
All data were compiled in Excel and analyzed using Qlikview (8.50) software. For analysis of the two factors, an unpaired Student t-test assuming unequal variances was used to compare the three outcomes between the appropriate teams. All analyses were performed using Microsoft Excel 2007 for Windows and statistical significant at p<0.05 was assumed.
Results
Outcome
A total of 100 individual Med Recs were observed. The PDSA cycle was repeated three times for each of the three teams piloting the intervention. Between two observers, nine bed-rounds were observed with nine different attending physicians.
All three medical teams operated in a similar workflow process. Each member of the team had a specific role during morning bed-rounds. The attending physician was responsible for leading the discussion on the patient and teaching residents. One of the two residents was responsible for reading the updated status on their patient. The physician assistant or nurse practitioner was responsible for ordering medications and procedures on the mobile computer. In this setting, the Med Rec review through the mobile computer was effective. The prescriber on the mobile computer (usually another resident or physician assistant) had fewer responsibilities during the discussion compared to the resident in charge of the patient.
Med Rec added an average of only 49 seconds to each patient’s bed-rounding time.
Completeness of Med Recs: Name, Dose, Frequency, Route
Despite education, not all prescribers consistently practiced complete Med Rec. Time pressures led the medication review process to become less comprehensive, and prescribers only read back the name and dose of each medication. Observers educated teams on the need to verbalize all four components of the Med Rec: name, dose, frequency, and route.
Environment and Medical Team Members
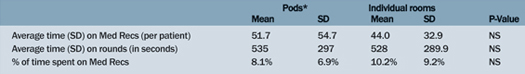
There were no significant differences among the teams when examining differences in environment and the members of the team (Tables 1 and 2). The only outcome reaching statistical significance was the unpaired t-test between the resident-driven team and the nurse-practitioner-team for average bed-rounding time. The resident teams (Team A and B) have a significantly longer bed-rounding time compared to the nurse practitioner team (Team C) (p=0.03).
Table 1. Environment: Pods Compared to Individual Patient Rooms
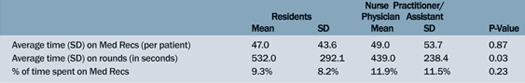
Table 2. Team Members: Residents Versus Nurse Practitioners/Physician Assistants
Changes and Clarifications
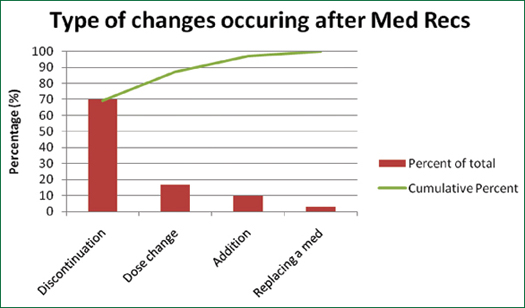
A formal review of active medications for each patient led to greater discussion and clarifications of discrepancies. A total of 30 changes out of a total of 100 patient medication lists were made among all three teams. A majority of medication changes were discontinuations (Figure 1 & Table 3, p. 20). Out of the changes, most medications were antibiotics or antifungal medications (Figure 2, p. 20). In total, 19 clarifications (i.e. questions or concerns answered after Med Rec) were made out of a total of 100 patient medication lists.
Figure 1. Types of Medication Changes
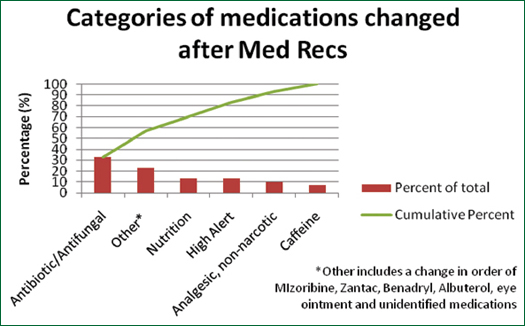
Figure 2. Categories of Medications That Were Changed
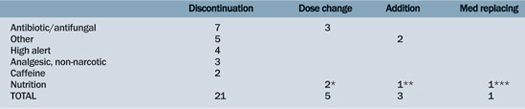
Table 3. Medication Categories Impacted by Medication Reconciliation
Discussion
This study supports the need for a formalized medication accountability system during an infant’s time in the NICU. The Med Rec tool shows potential in helping prevent adverse events in neonates. The Med Rec was especially effective in identifying medications that needed to be discontinued. This is important to avoid duplicate therapy, a common error in the NICU.
Although environment and team composition did not make a difference in the length of Med Rec per patient, the resident-driven teams spent a significant longer average time on rounds per patient than the nurse-practitioner-driven team. The teaching that occurs at the bedside for residents may contribute to this extended bed-rounding time. Yet despite residents spending more time by the patient’s bedside, there was no difference in the time it takes to practice Med Rec between the two teams. This may be due to two things: 1) Med Rec results in standardization across groups despite variable bed rounding time or 2) the Med Rec process is efficient and cannot be “leaned” any further. In other words, the Med Rec process cannot be trimmed of any more wasteful steps. If the latter is true, there may be a need for standardization of this “lean” Med Rec process.
To ensure completeness of this medication review practice, prescribers read-out the name, dose, frequency, and route of each medication. Even without all four components, the Med Rec process increased awareness of medications and clarified medication order discrepancies. Identifying these medication errors that were not originally detected by CPOE led to improved communication among clinicians.
Challenges to Medication Reconciliation
As past studies have shown, staff compliance is a significant barrier due to the intervention not being perceived as a part of regular care (Hall, 2010; Coffey et al., 2009). Even with education and physician agreement on the principles of reviewing medications, it was difficult to achieve full compliance due to the culture of the unit. Both observers agreed that there was a common perception among medical teams that the Med Rec process was extra work and time. Constant education during rounds was necessary to remind the team to go through the medication list before moving on to the next patient. Although this varied with each attending physician, we found there was a steady decrease in compliance when time was limited and teams sought to move on to the next patient.
Factors for Success
The practice of reviewing medications depended heavily on the attending physician and his/her role in initiating Med Rec. A clear physician champion within the team contributed to greater practice of Med Rec. A physician champion can be defined as a local leader for a quality improvement initiative. This is consistent with past literature showing physician engagement as an essential component to any quality improvement initiative (Walsh et al., 2009). In cases where there was a physician champion, the Med Rec process was done for nearly every patient. Interestingly the champion was sometimes the frontline clinician who insisted on completing medication reconciliation before moving on.
In addition, the most up-to-date list of active medications per patient was important in an accurate reading of medications for each patient. In line with past studies, using electronic ordering systems proved more reliable in providing the most up-to-date and comprehensible medication list compared to written notes of residents. The CPOE system played a central role in reviewing medications since medication lists are frequently updated and weight-adjusted for the neonate patient. The use of a mobile computer was a key element in practicing Med Rec during bed-rounds.
Future Research
Although past studies have shown medication reconciliations to be effective at admission and discharge, they may appear to be useful in daily bedside rounds during a patient’s non-transfer stay. Further research needs to be done in this area in other hospital settings. It is unknown which method is most effective in delivering a formal medication accountability process in other hospital units with a different workflow process. Moreover it is unknown if physicians are the best clinicians to initiate the Med Rec process due to their multifaceted role in the unit. Nurses or clinical pharmacists as initiators of the Med Rec process may result in a greater compliance.
Another domain to address is developing a system to identify patients at risk for medication-related adverse events and targeting daily medication reviews in this population. Creating an “alert system” which identifies risk factors (e.g. high-alert medications) related to medication errors may identify patients who will benefit the most from the accountability system.
Limitations
The biggest limitation in our study was the Hawthorne effect. The Hawthorne effect can be defined as the increased compliance due to the presence of an outside observer. Since observers were responsible for education, medical teams may have felt a greater need to comply to the Med Rec process simply because of the presence of an observer. Another limitation is the fact that not all clarifications and changes may be recorded since both could happen after bed-rounds when the observers were not present.
Conclusion
In summary, a physician-led medication accountability system may be an effective way to reduce common medication errors in the NICU. Many barriers still exist for a daily Med Rec system and further research needs to be done to prove its effectiveness during morning bedside rounds. For successful implementation of the Med Rec process, there are a few important aspects to consider. First, the development and implementation of Med Rec should be driven by patient safety and patient-centered care. If physicians and health providers do not grasp this concept, there will be a lack of physician leaders, which may lower compliance in the implementation process. In order to reach this level of awareness, it is important to practice ongoing education and feedback on the benefits of Med Rec for neonatal patients.
The authors work in the division of neonatology at Children’s Hospital of Philadelphia, Pennsylvania.
Sanghee Suh is the clinical research coordinator and may be contacted at sghee.suh@gmail.com.
Melody Linton is the student researcher and may be contacted at melodymlinton@gmail.com.
John Chuo is the neonatal quality informatics officer and may be contacted at chuoj@email.chop.edu.
References