 |
 |
 |

January / February 2007


Smart Pumps:
Advanced Capabilities and Continuous Quality Improvement
By Tim Vanderveen, PharmD, MS
The introduction of "smart" (computerized) intravenous (IV) infusion pumps in 2001 signaled a major advance in medication safety. For the first time, pumps with safety software could automatically alert clinicians to avoid IV infusion programming errors that otherwise could have tragic results. Since then, both devices and software have continued to evolve. Smart pumps' capabilities to help avert high-risk medication errors remain a major reason for their high rate of implementation (Pederson, 2006). However, the value of the continuous quality improvement (CQI) data automatically collected by the pumps is increasingly recognized, as hospitals use the data to help achieve significant best practice, quality, and financial improvements. This article reviews the need for improved safety of high-risk IV infusions, recent advances in smart pump capabilities, and examples of how CQI data can be used to help improve medication safety and best-practice compliance.
Need for Safer IV Infusions
The recent release of the Institute of Medicine's (IOM) report, Preventing Medication Errors, once again raised awareness of the frequency and severity of medication errors (IOM, 2006). This latest in the IOM's series of reports makes clear that with regard to medication errors, there is still a long way to go. The IOM found that medication mistakes injure more than 1.5 million patients each year and that hospitalized patients are at risk for at least one medication error per patient day (IOM, 2006).
Administration of medications via the IV route often results in the most serious outcomes of medication errors (Hicks, et al., 2003). Anticoagulants, thrombolytics, narcotics, hormones, and sedatives are among the highest-risk-of-harm medications (Maddox, et al., 2006), and 60% of the most serious and life-threatening potential adverse drug events are related to IV infusions (D.W. Bates, personal communication, 2001). In one 8-hour period during which every active IV infusion was evaluated just one time, 67% had one or more errors (Husch, et al., 2005). A United Kingdom study that observed the preparation and administration of IV medications found a 49% error rate, including 70% of the IV bolus doses administered too fast (Taxis & Barber, 2003). A recent intensive care unit (ICU) study found 47% of adverse events involved medications, and wrong dosages were the most common errors (Rothschild, et al., 2005).
IOM Recommendations
The IOM report (2006) identified a number of strategies, practices, and technologies that hospitals can employ to help reduce the frequency and severity of medication errors. Among these is the adoption of smart infusion pumps.
A recent report of technology adoption for improved medication safety documented that 37% of hospitals are now using smart infusion pumps (Pederson, 2006). This adoption rate is much higher than for other technologies recommended by the IOM, specifically bar code medication administration (BCMA) at 13.2% and computerized prescriber order entry (CPOE) at 8.9% (Pederson, 2006). Factors that have accelerated the rapid adoption of smart pumps include the recognition of the high rate of harm noted above, and findings that only 2% of medication errors in the administration phase are intercepted and that many patients receive IV infusions of high-risk medications in non-critical-care settings. (Leape, et al., 1995; Williams, et al., 2006).
Continuing Evolution of
IV Infusion Safety
When infusion pumps were first introduced to patient care in the early 1970s, their use was typically reserved for IV nutrition in general care and cardiovascular drug infusions in intensive care units (ICUs). The first infusion pumps were general-purpose devices with limited capabilities and applications. Over the past 35 years, the use of infusion pumps for an increasingly wide range of medications and other fluids has become a standard throughout the world.
Numerous factors have created the need for these pumps to be used in all patient care areas, including increasing patient acuity, high-risk infusions in general care areas, and therapy-specific infusions such as patient-controlled analgesia. Infusion pumps provide accurate delivery over a wide range of rates, warn of conditions such as occlusions and empty containers, and provide protection against inadvertent free flow when the pump tubing is removed from the device.
Despite these safeguards, inaccurate programming of general-purpose infusion pumps has resulted in tragic outcomes. To improve IV safety, computerized infusion pumps known as "smart pumps" with dose error reduction systems (DERS) were developed to provide immediate feedback if a programmed infusion dosage exceeds the hospital's best practice guidelines.
While early-generation smart pumps focused on a limited number of continuous infusion medications, the capabilities of these devices have rapidly expanded to cover virtually every IV infusion (Table 1).
|
Advanced Capabilities
Additional Safety Alerts
Drug Selection by Therapeutic Indication
Patient-Controlled Analgesia (PCA)
Bar Code Medication Administration
|
|
Smart infusion pumps are not shipped to hospitals with the DERS software already installed. Ideally this could simplify adoption; however, the high degree of variability in IV infusions both within and between hospitals makes this both impractical and potentially dangerous (Bates, et al., 2005). Rather, each hospital must create its own library to match its current practices with IV medications. Customized drug libraries are created for specific patient care areas (profiles), such as ICU, pediatric ICU (PICU), neonatal ICU (NICU), labor and delivery, etc. Each smart pump is capable of functioning in all care areas: the clinician simply selects and confirms the appropriate drug library at each power-on of the device.
Care area profiles are customized to match the best practices for the drugs used in each area. A menu of IV drugs is listed by brand or generic names and standard concentrations. Selecting the drug and concentration (if more than one concentration is available) automatically selects the correct dosing units and populates these values in the smart pump's drug dose calculator.
The drug library both simplifies programming of IV infusions and provides new levels of safety. For example, if dopamine is programmed to infuse at 50 mcg/kg/min and the high-dose limit is 20 mcg/kg/min, an alert is immediately posted that the programming exceeds the maximum recommended dosage. The alert must be addressed before infusion can begin. Dosage alerts can be soft (can be overridden) or hard (must be reprogrammed), depending on how a hospital sets up the library.
In keeping with the Joint Commission on Accreditation of Healthcare Organizations' National Patient Safety Goal 3B, which requires hospitals to standardize and limit the number of drug concentrations available in the organization (JCAHO, 2005), drug library standard concentrations are recommended for continuous infusions such as cardiovascular drugs (e.g., dopamine, nitroprusside). Intermittent infusions including thrombolytics and chemotherapy are typically prepared individually for each patient, based on patient weight or BSA. When patient-specific concentrations are dispensed by pharmacy, the concentration is programmed by the individual caregiver, and limits around the concentration used can help to prevent data-input errors.
For example, if a pump were programmed with an incorrect, weak concentration for dopamine of 40 mg/250 mL, instead of the correct concentration of 400 mg/250 mL, in order to achieve the ordered dosage the pump would increase the rate, which would result in a potential 10-fold overdose. A drug library with a standardized concentration for dopamine (400 mg/250 mL) eliminates the potential for this error. Similarly, if a hospital has standardized mcg/min dosage units for all nitroglycerin infusions, it is not possible to administer this high-alert drug as mcg/kg/min. For dopamine, nitroglycerin, and many other standardized infusions, both the concentration and dosage units are automatically programmed when the drug is selected.
Value to Clinicians and Hospitals
For the individual clinician, the immediate value of smart pumps comes from both the reduction in programming steps required to initiate an IV infusion and the alerts that help to prevent inadvertent infusion administration errors. These alerts can help save a patient's life and help prevent what could be a career-ending mistake for the clinician.
The first article published on smart pump use reported smart pumps' immediate, positive impact on safety. A potentially life-threatening error was identified during the switchover to the smart IV pumps at the initial go-live (Eskew, et al., 2002). A physician had written an order for Integrilin (eptifibatide) but inadvertently prescribed a dose appropriate for ReoPro (abciximab). The error was averted due to the alert generated when the new smart pump was programmed exactly as the old pump being replaced had been. Following the first 3 months of use, an assessment of smart pump data suggested that as many as 1,000 programming errors were averted in this 750-bed hospital. Despite a voluntary error reporting system, the averted errors had not been reported by the individual caregivers.
Examples of reported patient deaths that could have been prevented by smart pumps with DERS include:
- Morphine infusion programmed at 90 mg/hr (intended rate was 9 mg/hr);

- Heparin volume to be infused programmed as the rate: infused at 500 mL/hr (intended dosage was 14mL/hr);

- Nitroglycerin ordered administered as mcg/kg; infusion programmed as mcg/kg/minute.
In the cases of the morphine and heparin overdoses, the clinicians would have received alerts that would have required reprogramming before infusion could begin. In the case of the nitroglycerin infusion where the calculation error involved using the patient's weight to calculate the dose, there would not have been an alert, since the smart pump simply would not have allowed the use of weight-based programming. Thus, there are two types of averted errors — programming errors (most typically dose, rate, or duration) that are prevented because they fall outside of the limits set by the DERS, and those that can no longer occur because they are prevented by the best practices built into the smart pump's safety software.
The value of smart pumps typically is measured by the alerts that resulted in reprogrammings. The pumps' standardized concentrations and dosing units can be assumed to have prevented errors similar to those reported in the past. However, since these errors can no longer be made, it is very difficult to measure this aspect of smart pump value.
Numerous reports show that the purchase and implementation of smart pumps with DERS is having the desired impact of improving patient safety (Eskew, 2002; Wilson & Sullivan, 2004; Hatcher, et al., 2004; Malashock, et al., 2004; Daniels & Rapala, 2005; Fields & Peterman, 2005; Williams, et al., 2005; Maddox, et al., 2006; Williams, et al., 2006; Cassano, 2006) However, some smart pumps provide the added value to hospitals of incorporating CQI logs in the pumps' software to automatically collect data that can be used to document the impact of the pumps on medication safety and to identify opportunities for best practice improvements.
CQI Logs: Window to
IV Infusion Practices
The previously unavailable data now gathered by smart pumps provide a new "window" to IV infusion practices. The software's CQI logs automatically document every alert, the medication involved, initial dose programmed, and the subsequent action by the caregiver. In addition, the logs document the patient care area or patient type, location, time, and date. Where the patient-identification option is implemented, the logs can also capture patient-specific events. In this way the CQI logs provide a "treasure trove" of new information (Bates, 2004) to measure the impact of these devices, evaluate how physician's orders are actually carried out, and identify and implement best practice and compliance improvements.
When smart IV pumps were first introduced, accessing the pump logs required connecting every device to a laptop computer to download the CQI data. In most cases, the data were downloaded during 6-month or one-year preventative maintenance by the biomedical engineering department when a device was returned to a central area for cleaning. A significant advance has been automating data collection through the use of wireless connectivity between a hospital's information systems' network and each smart infusion device.
A rapidly increasing number of hospitals now have wireless connectivity to each smart pump. Prior to such connectivity, one hospital system's first look at CQI data covered an 8-month period and included over 8,000 alerts. Now CQI data are collected automatically and reports are generated for review by pharmacists, nurses, and risk managers on a daily basis. Practice issues can be identified quickly, dose limits that might be causing nuisance alerts can be adjusted, and drug information advisories can be posted to rapidly communicate new information. Collecting the data, creating the information, and acting on the findings now happens in days compared to months and years prior to the wireless systems.
Converting smart pump data into useful CQI information is facilitated by software programs, training, and services provided by smart pump manufacturers. The software, which is unique for each smart pump system, can typically generate 15 or more separate reports (see Figure 1 on p.44). These reports help individuals and departments identify high-risk practices, the drugs most associated with programming issues, and current practice issues that may need realignment. For example:
- The hospital's administration and hospital board might be most interested in trends across the entire hospital or hospital system, such as decreases in the number and rate of high-risk IV medication errors that document improved medication safety.

- The data generated in pediatrics on the drugs most frequently associated with errors and the type and magnitude of those errors will be of most interest to the nurse manager of the pediatric units.

- Members of the medical staff may have special interest in the overrides of high-dose alerts, as they evaluate how their medication orders are actually implemented.
With a variety of reports available, each interested party can focus on those aspects of the data that are of most importance to their area of responsibility.
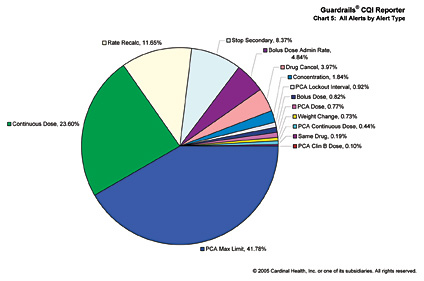

Smart pump data logs are now a rich source of information that hospitals can use to determine the frequency of averted errors and identify opportunities for continuous quality improvement (CQI) efforts. Since in many cases collection, interpretation, and application of the CQI data is a new responsibility for staff in one or more departments, some smart pump manufacturers offer extensive support services for education and training. In addition to clinical pharmacists who help hospitals create and maintain the drug libraries, nurses frequently work with individual hospitals' CQI data. One manufacturer has created a novel "CQI University" program, including highly experienced faculty from hospitals aggressively using their CQI data, to enhance the analytical skills of clinicians new to this process.
Averted Errors
Pooled data, initially from 7 hospitals, then 10, 18, and now 32, have shown a consistent level of averted harm from programming errors. The rate of averted serious to life-threatening errors involving 2.3 million patient days of IV infusions is approximately 0.8 events per 1,000 patient days. Less severe but still significant programming errors that could have resulted in moderate harm are also approximately 0.8/1000 patient days, for a combined rate of 1.6/1,000 patient days (Cardinal Health, 2006).
An important finding in the pooled CQI data is the even distribution of averted errors between ICU's and medical/surgical units (Cardinal Health, 2006; Williams, et al., 2006). Many high-risk medications, including anticoagulants, narcotics, electrolytes, and hormones such as insulin and steroids, are routinely administered to patients in general care units. As a result, smart pumps typically are implemented throughout a hospital in all patient care areas.
In the 10-hospital pooled CQI data the percentage of averted errors in the ICUs and in the non-critical-care units were similar (Cardinal Health, 2006). In a tertiary-care health system, even though smart pumps were used most often with ICU patient types, slightly more than half of the highest-risk averted overdoses were associated with non-ICU patient types (Table 2) (Williams, et al., 2006). This very significant finding suggests that without smart pump use, the risk for patient harm due to IV medication errors may actually be greater outside the ICU, based on the lower likelihood of detection and less frequent monitoring.
When hospitals take their first look at their CQI data, often they are surprised by the high frequency of alerts that result in overrides. An override, which can only follow a "soft" alert, indicates that an alert did not result in reprogramming by the clinician. Overrides may result from dose-limit alerts that have been set too low, but more frequently overrides represent a conflict between a hospital's best practice guidelines and current practice. The clinician does not perceive that an override is dangerous or associated with an error, and in most cases current practice wins out over new best practices.
For example, in the ICU sedative drugs such as propofol or midazolam are typically administered as continuous infusions, but frequent boluses are administered to rapidly increase the level of sedation. A common practice is to increase the infusion rate to 250, 500, or even 1,000 mL/hr to administer a bolus dose from the continuous infusion container. An ICU patient attempting to remove a feeding tube or get out of bed may require more sedation. Frequently these high rate infusions are not programmed with a limited infusion volume, but rather are administered until the undesirable activity subsides.
This has been a common practice prior to the implementation of smart IV pumps, and the practice often continues, at least initially. An alert that a dose is too high simply results in an override. Analysis of the CQI data on overrides often reveals practices that over time have become "acceptable" and common but that deviate from hospital policy and need to be addressed. In the example above, the administration policy could be modified to require use of the bolus dose feature in the smart pump, resulting in limits on the amount of each bolus dose.
Risk Reduction
Failure mode and effects analyses (FMEA) were performed at a tertiary-care health system both before and after smart pump implementation. Results showed that the risk priority score related to setting IV heparin infusion rates decreased from 210 to 56 — nearly a four-fold reduction in risk as a result of having implemented smart pumps with DERS (Fields & Peterman, 2005).
Process Improvements
Collection and analysis of the CQI data has resulted in numerous examples of changes that led to improved safety, better compliance, and reduced patient risk. Among such process improvements are the following examples:
- One hospital system identified a high frequency of bolus doses of propofol administered by turning up the rate of continuous infusions. The practice involved overriding the high-dose alert, and the volume to be administered was not set. Several issues were identified, new standard orders were created, bolus dose limits were incorporated into the pump library, and nurses were educated on the use of sedation scales. ICU drug costs, length of stay, and ventilator-associated complications were all dramatically reduced (Table 3; Maddox, 2005).

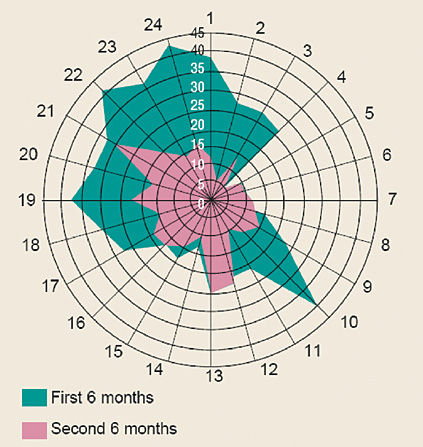
- A chronogram developed from time-based data at a children's hospital revealed that infusion programming errors — instances in which a clinician reprogrammed an infusion in response to a safety software alert — occur in identifiable patterns. As shown in Figure 2, errors peaked significantly during busy times such as shift change, high admission volumes, and activities requiring drug distribution. As a result of these findings, the units were closed during shift change, and the timing of several elective activities was changed to reduce distractions. Following implementation of these changes, infusion safety alerts were much less frequent and all of the former error peaks were eliminated (Billman, 2004).

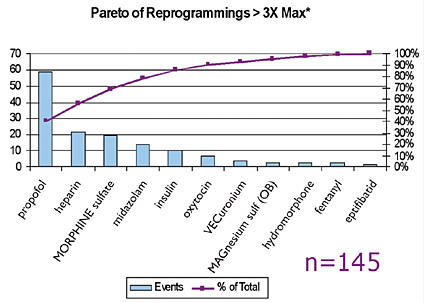
- A multi-hospital system sorted all averted errors based on how many times over the limit they initially were programmed to infuse. All instances of excessive doses of drugs such as vasoactive drugs that could have been identified by intensive care monitoring were removed from the data. In the remaining data, all instances where initial programming was more than three times over the limit and a clinician subsequently reprogrammed the pump in response to an alert were considered "good catches," i.e., safety benefits gained from use of the pump. The data showed that in a 3-month period, the use of smart pumps helped avert 145 serious errors that otherwise most likely would have reached a patient (Figure 3) (N. Pratt, personal communication, 2006).

|
Changes After ICU Sedation Protocol Implemented
|
|

Conclusion
Computerized infusion safety systems, i.e., smart pumps, are one of the technologies identified in the recent IOM report (2006) that hospitals can employ to help reduce the frequency and severity of medication errors. The rate of adoption of smart pumps is approximately three to four times that of BCMA and CPOE, respectively. Since the introduction of the first smart pumps in 2001, both devices and safety software have continued to evolve. Smart pumps provide immediate benefit in helping to avert potentially serious and life-threatening medication errors. In addition, smart pump CQI data and customized reports can help staff to address previously unrecognized medication safety issues, refine therapeutic regimens, improve compliance, and identify opportunities for best practice improvements. In selecting and implementing smart pump technology, hospitals need to evaluate advanced capabilities, CQI data reports, and the training and support programs to help hospitals realize both the immediate and long-term safety, clinical, operational, and financial benefits of smart pump use.
Tim Vanderveen is currently the vice president of the Cardinal Health Center for Safety and Clinical Excellence. From 1972 to 1982, Vanderveen was the director of clinical pharmacy at the Medical University of South Carolina. Since 1983, he has been in the medical device industry, with IMED, Alaris, and most recently Cardinal Health. He has been instrumental in many of the safety and performance enhancements and safety innovations in drug infusion. Vanderveen has authored numerous patents, is a frequent speaker on medication safety at educational programs, and routinely contributes to the medical, pharmacy, and nursing literature. He may be contacted at Tim.Vanderveen@cardinal.com.
References
Bates, D.W. (2004). Introducing breakthrough technology with immediate impact in preventing harm to patients. VHA-TV, April 6.
Bates, D.W., Vanderveen, T., Seger, D.L., et al (2005). Variability in intravenous medication practices: implications for medication safety. Joint Commission Journal of Patient Safety and Quality, 31(4), 203-210.
Billman, G. (2004, December). Evidence-based improvements prevent high-risk errors. Harm isn't random. Nursing Management, (Supplement), 7-8.
Cardinal Health (2006). Data on file.
Cassano, A.T. (2006). IV medication safety software implementation in a multihospital health system. Hospital Pharmacy, 41(2), 151-156.
Daniels. D, Rapala, K. (2005). Aiming for zero errors. Clarian's safe passage program improves safety. Patient Safety & Quality Healthcare, July/August, 14-20.
Eskew, J.A., Jacobi, J., Buss, W., et al. (2002). Using innovative technologies to set new safety standards for the infusion of intravenous medications. Hospital Pharmacy, 37, 1179-1189.
Fields, M,, Peterman, J. (2005). IV medication safety system averts high-risk medication errors and provides actionable data. Nursing Administration Quarterly, 29(1), 77-86.
Hatcher, I., Sullivan, M., Hutchinson, J., Thurman, S., & Gaffney, F. A. (2004). An intravenous medication safety system: preventing high-risk medication errors at the point of care. Journal of Nursing Administration, 34(10), 437-439.
Hicks, R. W., Cousins, D. D., & Williams, R. L. (2003). Summary of information submitted to MEDMARX in the year 2002. The quest for quality. Rockville, MD: USP Center for the Advancement of Patient Safety.
Husch, M., Sullivan, C., Rooney, D., et al (2005). Insights from the sharp end of intravenous medication errors: Implications for infusion pump technology. Quality and Safety in Health Care, 2005, 14(2), 80-86.
Institute of Medicine (2006). Preventing Medication Errors: Quality Chasm Series. Washington, DC: National Academies Press. PDF available at: http://www.nap.edu/catalog/11623.html
JCAHO (2005). Hospitals' National Patient Safety Goals. http://www.jcaho.org/accredited+organizations/hospitals/npsg/05_npsg_hap.htm
Jeffrey, M., Rothschild, J.M., Landrigan, C.P., et al (2005). The Critical Care Safety Study: The incidence and nature of adverse events and serious medical errors in intensive care. Critical Care Medicine, 33(8), 1694-1700.
Leape, L.L., Bates, D.W., Cullen, D.J., et al (1995). Systems analysis of adverse drug events. Journal of the American Medical Association, 274, 35-43.
Maddox, R.R. (2005). ICU Sedation with propofol: measuring and reducing variation. In: Schneider PJ (ed). Sedation Therapy: Improving Safety and Quality of Care. 35-38.
Maddox, R.R., Williams, C.K., Oglesby, H., et al. (2006). Clinical experience with patient-controlled analgesia using continuous respiratory monitoring and a smart infusion system. American Journal of Health-System Pharmacists, 63, 157-164.
Malashock, C.M., Shull, S.S., Gould, D.A. (2004 May). Effect of smart infusion pumps on medication errors related to infusion device programming. Hospital Pharmacy, 39(5), 460-469.
Pedersen, C.A. (2006 December). Patient safety technologies. In: Scheckelhoff DJ, Schneider PJ, Pedersen CA. Results from the 2006 National Survey of Pharmacy Practice in Hospitals and Implications for the ASHP 2015 Initiative. Presented at ASHP Mid-year Meeting, December, Anaheim, CA.
Rothschild, J.M., Keohane, C.A., Cook, E.F., et al. (2005). A controlled trial of smart infusion pumps to improve medication safety in critically ill patients.* Critical Care Medicine, 33(3), 533-540. (*See also pg 679.)
Taxis, K., & Barber, N. (2003). Ethnographic study of incidence and severity of intravenous drug errors. British Medical Journal, 326(7391), 684.
Vanderveen, T. (2006). IVs first — a new bar-code implementation strategy. Patient Safety and Quality in Healthcare, May/June, 14-20.
Williams, C., Maddox, R. R. (2005). Implementation of an i.v. medication safety system. American Journal of Health-System Pharmacists, 62, 530-536.
Williams, C.K., Maddox, R.R., Heape, E., et al (2006). Application of the IV Medication Harm Index to assess the nature of harm averted by ''smart'' infusion safety systems. Journal of Patient Safety, 2, 132-139.
Wilson, K. & Sullivan, M. (2004). Preventing medication errors with smart infusion technology. American Journal of Health-System Pharmacists, 61, 177-83.
|
 |
 |
 |


















|
 |



