
September / October 2006

Heart Safe or Heart Break: What Every Clinician Needs to Know About AEDs

By Laury A. Sendek
Cardiac arrest is a leading cause of death in the United States. The American Heart Association estimates that this year 250,000 Americans will die of sudden cardiac arrest before reaching a hospital. That's 680 deaths each day; one death every 2 minutes.
Sudden cardiac arrest usually occurs without warning and can strike people of all ages, even physically fit, apparently healthy adults and children. Eighty percent of cardiac arrests are caused by ventricular fibrillation (VF), an abnormal heart rhythm that prevents the heart from pumping blood effectively. The only treatment for VF is immediate electrical heart defibrillation.
When cardiac arrest occurs outside of a hospital, defibrillation is administered through an automated electronic defibrillator (AED). An AED is a lifesaving medical device that evaluates the cardiac status of a person who is suspected of suffering from cardiac arrest. It contains a microprocessor that analyzes the heart's rhythm for abnormalities and, if necessary, guides the user through the process of administering a defibrillation shock. This shock causes all of the muscles in the heart to contract with the goal of jolting it out of the fatal rhythm and restoring a more natural, healthier heartbeat.
AEDs are easy to operate and can be used with minimal training. Once turned on, an AED instructs the user to place the pads and electrodes on the victim's chest then issues a series of verbal and visual instructions to guide the user through the defibrillation process.
Time is the most important factor in a sudden cardiac emergency. The odds of survival decrease 7% to 10% for every minute without immediate cardiopulmonary resuscitation (CPR) and defibrillation. After 10 minutes without defibrillation, few resuscitation attempts are successful (AHA). Administering CPR while waiting for a defibrillator to arrive can increase a victim's chance of survival because it keeps the blood flowing to the heart and brain. CPR is also important immediately following defibrillation until the heart is able to pump effectively. The American Heart Association (AHA) estimates that at least 20,000 lives can be saved annually by delivering defibrillation with AEDs (National Conference of State Legislatures).
Recalls, Failures, and Other Dangers
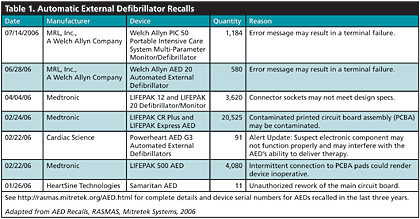
The good news is that ready-access to AEDs saves the lives of thousands of cardiac arrest victims every year. The bad news is that many AEDs — more than 100,000 — have been recalled in the last 3 years. More than 30,000 AEDs have been recalled in 2006 alone. This trend in defibrillator recalls was noticed by Risk And Safety Management Alert System (RASMAS) analysts at Mitretek Systems, who compiled a list of 103,553 AEDs that have been recalled since the beginning in Sept. 2003 (See Table 1). Full details of these recalls, including device serial numbers, are available http://rasmas.mitretek.org/AED.html.

|



