 |
 |
 |

September / October 2005

Barcoding and RFID
Barcoding to Enhance Patient Safety
By Joseph Cummings, PhD; Thomas Ratko, PhD;

Karl Matuszewski, MS, PharmD
The National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) defines a medication error as "...any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer" (2004). By definition, these errors can occur at any stage in the medication process, which includes prescribing, order communication, product labeling, packaging, compounding, dispensing, distribution, and administration. Various information technologies have been applied to reduce errors throughout the medication process (Chung et al., 2003; Oren et al., 2003; Bates, 2000), including computerized prescriber order entry (CPOE), robotics, automated dispensing devices, and the electronic medication administration record (eMAR). This article focuses on barcode technologies applied at the administration stage of the process.
Research has shown that errors that occur earlier in the medication process are more readily detected (~50% are prevented during the ordering stage) while very few (< 2%) are caught at the administration stage (bates et al., 1995). further, it has been noted that more than one third of medication errors occur at the latter stage (leape et al., 1995). because of the relatively high proportion of errors and the lack of success preventing them, error reduction strategies targeted at the administration stage have recently received significant attention from providers, specialty societies, and regulatory agencies.
An emerging information technology designed to address medication administration errors uses barcodes on both the medication and patient that are scanned at the point-of-care (POC) before administration. At its most basic level, barcode-assisted prevention of medication administration errors is predicated on verifying the "5 rights"; the right patient, right drug, right dose, right route, and right time.
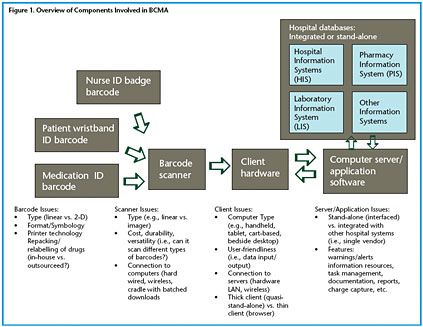
A summary of the barcode-assisted process for medication administration is shown in Figure 1. As can be seen, the barcoded medication administration (BCMA) technology is comprised of a number of hardware and software components that may include a barcode printing system for both wristbands and medication packages in the pharmacy; portable barcode scanners interfaced to computers located near the patient; a communications network (usually wireless) tying the portable bedside computers to a centralized computer server; software applications running on the server that process the incoming information and return information to the bedside computers; and interfaces for communication between the server/software and other hospital databases, such as the pharmacy information system and the admission discharge transfer (ADT) system.

|
 |
 |
 |


















|
 |













