 |
 |
 |

January / February 2009

Heparin: Improving Treatment and Reducing Risk of Harm
Clinical, Laboratory and Safety Challenges

William E. Dager, PharmD, FCSHP
Robert C. Gosselin, CLS
Robert Raschke, MD, MS
Tim Vanderveen, PharmD, MS
The short-acting, reversible anticoagulant heparin is widely used in hospitalized patients to prevent the development or extension of potentially life-threatening blood clots. However, numerous issues make the use of this high-risk agent particularly challenging and error-prone. As shown by media reports of heparin-related infant deaths and injury in Indiana, California, and Texas, heparin-related medication errors can have devastating impact on patients, families, staff, and the reputation of a healthcare institution and its leadership.
Safe and effective use of heparin requires maintaining a delicate balance — dosing low enough to minimize the risk of bleeding, yet high enough to treat or prevent thrombosis. Achieving a therapeutic level of heparin within 24 hours significantly reduces the risk for recurrent venous thromboembolism (VTE) (Raschke et al., 1993; Hull et al., 1997; Anand et al., 1996; Anand et al., 1999). However, non-protocol-driven practice achieves this outcome only 40% of the time (Wheeler et al., 1988).
Medication errors involving unfractionated heparin (UFH) are among the most common and serious in clinical practice. More than 17,000 heparin-related medication errors were reported to the U.S. Pharmacopoeia (USP) MEDMARX from 2003 to 2007; 556 of these resulted in harm to patients, including seven deaths (Santell, 2008).
An expert advisory panel of The Joint Commission (TJC) recognized that "anticoagulation is a high-risk treatment which commonly leads to adverse drug events because of the complexity of dosing, monitoring and patient compliance" and that "the use of standardized practices can reduce the risk of adverse drug events." In 2008 TJC issued National Patient Safety Goal 3E (NPSG 3E) to "Reduce the risk of harm associated with the use of anticoagulant therapy." Hospitals were required to be fully compliant with this goal by January 1, 2009 (TJC NPSG, 2008).
Key clinical, laboratory, and safety issues surrounding heparin were addressed in a nationwide webcast on "Improving Heparin Safety," hosted by the Center for Safety and Clinical Excellence on July 11, 2008. In this article, we summarize important information and recommendations, focusing on the three stages of heparin use that account for 67% of heparin-related errors — dosing, monitoring and administration (Santell, 2008).
Dosing
Significant errors in dosing heparin and interpreting reported aPTT values can result from unnecessary variation in physician practice. Standardized protocols for dosing heparin can help reduce such variability. Although physicians sometimes are perceived as being resistant to change, in fact they are more resistant to having change mandated. For this reason, prescribers need to participate in the development and adoption of heparin-related standards, guidelines or protocols.
The Deep Vein Thrombosis Prevention program at the University of California, San Diego Medical Center used a multidisciplinary consensus-building process to develop and implement a standardized, physician-friendly, risk-assessment process and VTE-prevention order set. Results showed that appropriate VTE prophylaxis increased from 55% to over 95% of patients and was accompanied by a documented decrease in thromboembolic events (Peterson et al., 2008).
Approaches to initiating heparin infusion to reach targets were also reviewed. A study by Raschke et al. showed that compared to a standard, "one-size-fits-all," initial heparin dosing scheme, a weight-based protocol increased the percent of patients within the therapeutic range within 24 hours from 77% to 97% (p=0.002) and significantly reduced the risk of recurrent venous thromboembolism (RR 0.2, p=0.02). The incidence of bleeding complications was the same in both groups, despite more aggressive dosing in the weight-based group (Raschke et al., 1993).
A subsequent study showed that improvements were maintained over a 5-year period with a nearly 95% protocol implementation rate. Initial heparin infusion rates increased from 1,185 to 1,420 units/hour (p<0.001), and the mean time to achieve a therapeutic aptt decreased from 19.6 to 11.8 hours (p<001) (Raschke et al., 1996). however, efforts to generalize the use of the weight-based protocol in other institutions have been hindered by the significant variability in aptt monitoring.
Monitoring
Successful monitoring depends on the degree to which aPTT results correlate with serum heparin levels. Numerous factors can adversely affect accuracy, including variability in instrumentation and sensitivity of aPTT reagents, pre-analytic variables, and errors in assessing the impact of initial bolus dosing on aPTT results. Point-of-care (POC) testing is an alternative to laboratory based testing, but these methods also are affected by analytical variables noted with laboratory methods. Lastly, there is increasing enthusiasm, but limited clinical data, for using heparin anti-Xa activity for monitoring UFH therapy.
The sensitivity of aPTT reagents may vary significantly between manufacturers (Favaloro et al., 2005) and even between lot numbers from the same manufacturer, especially over time (Table 1). More than 300 different instrument-reagent combinations are available, and the therapeutic ranges established using different systems and reagents vary widely (Raschke et al. 2003).
The College of American Pathologists (CAP) and American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy previously recommended that every institution independently validate its aPTT therapeutic range for new instrumentation/reagent changes and every reagent lot change (Dalen & Hirsh, 1995). A laboratory typically changes aPTT reagent every 12 to 16 months, depending on its manufacturer-determined stability. For example, following a change in thromboplastin reagent at Banner Good Samaritan Medical Center in Phoenix, Arizona, use of the CAP/ACCP-recommended recalibration method changed the therapeutic range for aPTT test results from 45-65 seconds to 70-105 seconds. This magnitude of change is not uncommon when a laboratory changes its thromboplastin reagent and can go in either direction. Failure to account for such change by updating heparin dosing schemes would have resulted in systematic underdosing of the vast majority of patients. For this reason, it is important that each institution adjust heparin dosing guidelines as necessary to the aPTT assay reagent currently in use.
Pre-analytical variables that adversely affect aPTT test results include poor phlebotomy technique, incorrect or improperly filled blood collection tubes, delays in sample testing, temperature, and inadequate sample centrifugation prior to testing. Some drugs and disease states can also affect test results (Table 2). Increased levels of fibrinogen and factor VIII (both acute phase reactants) may also reduce the aPTT result, leading to "heparin resistance."
Depending on the size of a heparin bolus, it can affect aPTT values for more than 6 hours. For example, 4 hours after a 5,000-unit bolus the aPTT result may suggest adequate heparinization, but a subsequent aPTT result may show that the continuous infusion is subtherapeutic. Earlier determination of aPTT value may be of benefit to determine if a rate increase should occur if the value is low.
Test results will vary among POC devices and typically do not match laboratory aPTT results, because of differences in the sample type, clot-detection method, and incubation period. There are also differences between ACT manufacturers and cartridge types. A different ACT test may be used depending on the desired degree of heparin effect is being followed, which can vary between methods. Use of high-dose ACT cartridges in patients with lower UFH infusion rates can lead to underestimation of UFH anticoagulation. The presence of a heparin effect may not be appreciated because the ACT test is not designed to measure effects in the lower target range. Thus, while POC testing devices provide rapid results, such devices may sacrifice accuracy and precision.
Most institutions that measure anti-Xa activity use either a clot based or a chromogenic method. While this test is more robust than the aPTT and may be less adversely affected by pre-analytical variables, test calibration variability and methodology differences can result in a wide range of anti-Xa levels reported, (CAP, 2008).
Administration
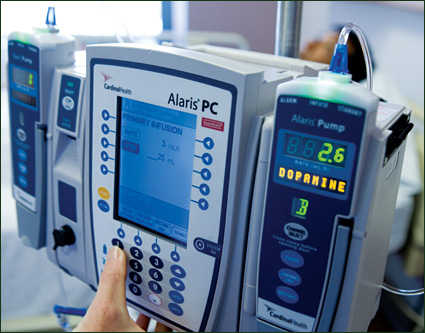
The most common type of heparin-related error is also the type most likely to be associated with patient harm — administration of an improper dose or quantity (Santell, 2008). Pooled data from 54 hospitals using smart IV pumps revealed that heparin was the number one drug associated with averted errors, i.e., smart pump alerts that resulted in reprogramming. Some errors, if not averted, would have infused dosages 50- to 100-times above or below the pre-established drug-library limits.(Cardinal Health, 2008) Analysis of smart-pump data at a regional healthcare system showed that 93% of high-risk heparin errors averted by smart pump use occurred in non-critical-care settings (Williams et al., 2006).
A frequent, potentially dangerous practice involves administering bolus doses from continuous infusions, rather than preparing a loading dose or subsequent bolus dose in a separate container such as a syringe or mini-bag. Serious errors can also occur in calculating dosage changes from an initial loading dose in units/kg to continuous infusion in units/kg/hr or units/hr.
Unnecessary variability in heparin concentrations, nomenclature and dosing units further increases opportunities for error. A review of smart-pump drug libraries in 207 hospitals identified 14 different heparin concentrations being used in various facilities. Heparin nomenclature also varied widely, with 191 different name-descriptors (Bates et al., 2005). Data from a 54-hospital sample showed that 48% of hospitals had standardized dosing units on units/kg/hr, 22% used only units/hr and 30% allowed either weight-based or non-weight-based dosing units. When both dosing units were available, there was a four-fold increase in smart pump alerts that led to infusion reprogramming, i.e., averted errors (Cardinal Health, 2008).
To reduce opportunities for such errors, NPSG 3E requires hospitals to standardize heparin concentrations. NPSG 3E also mandates the use of " programmable" infusion pumps, with a strong consensus among JC advisory panel members that smart pumps should be used for all heparin infusions (Peterson et al., 2008).
The introduction of barcode label scanning as a new smart pump-feature allows the manufacturer's barcode label on a pre-mixed heparin container to be scanned and the correct drug and concentration to be automatically selected from the pump library. Connecting the pumps to the hospital's wireless communication system allows rapid uploading of any needed drug-library changes and frequent downloading of data on averted programming errors and compliance.
St. Joseph's/Candler Medical Center in Savannah, Georgia, has used smart infusion pumps for more than 6 years. Smart pump continuous quality improvement (CQI) data were analyzed using a unique Harm Index to determine the potential harm of averted programming errors. Results showed that heparin administration in medical/surgical units posed the highest risk of harm for all IV infusions. To address heparin safety, the hospital system standardized IV heparin concentrations, streamlined the dose calculation process, eliminated the need for nurses and pharmacists to calculate infusion rates, standardized heparin dosing units, educated providers on revised dosing protocols (Williams et al., 2006), and implemented an inpatient anticoagulation management service whereby all UFH dosing is managed by pharmacy.
Special Cases
HIT is an uncommon but potentially devastating complication of treatment characterized by a drop in the platelet count to < 100,000/mm3 and/or to < 50% of baseline, typically after 4 to 14 days of heparin or low-molecular weight heparin exposure. two other onset patterns, delayed and rapid should also be considered (Dager et al., 2007). the decrease in platelets is due to the formation of antibodies that lead to platelet clumping. activation of the platelets leads to a hypercoagulable state and potential for thrombosis. bleeding secondary to reduced platelet counts is rare. failure to discontinue heparin and initiate alternative anticoagulation will result in thromboembolic complications in the majority of patients. hit-probability nomograms can be used to assess the likelihood that a patient has hit (Peterson et al., 2008; Janatapour et al., 2007).
Heparin errors in the pediatric population have recently been of notable concern and may be as or more common than in adults. Children vary greatly in size and weight and require different drug concentrations and dosage. Dosing in infants is particularly important, in part because there is little margin for error. Yet information on optimal drug use and how to adjust infusion or interpret laboratory data is scant, and support systems such as computerized physician order entry may be problematic. Children can be more difficult to monitor and often are unable to provide the sort of feedback that adults can. Additional commitment to children's safety is needed at every level of the healthcare community (Peterson et al., 2008).
Anticoagulation Service
As shown with pharmacists, having an anticoagulation service to oversee heparin therapy has been associated with a significant reduction in death, length of stay, cost of therapy, and bleeding complications (Bond & Raehl, 2004). An effective oversight team might include the responsible physician, bedside nurse, pharmacist, and laboratory technician. Overall, good working relationships need to be fostered among clinical, pharmacy and laboratory staffs, so that knowledge about heparin therapeutic-range determinations and coagulation testing is readily shared and disseminated.
After a series of serious and potentially tragic heparin errors, Fairview Health Services (FHS), a fully integrated, seven-hospital health system in the Minneapolis area, conducted a formal heparin failure mode effects analysis (FMEA) of the medication management use process. Based on the FMEA results, an FHS team took a three-pronged approach to improving heparin safety. This included revised storage and distribution of heparin, use of pre-typed protocols, a heparin dosing service, improved drug checking, use of smart pumps and barcoding, error detection using the anticoagulation management service, flow sheets, specialized software, and incorporation of anticoagulant-reversal protocols into dosing protocols. As a result of these actions, heparin errors decreased by 42% (Peterson et al., 2008).
A Heparin Error Reduction Workgroup at a large Minnesota healthcare organization examined heparin administration procedures, identified types and sources of errors, and developed solutions to reduce heparin errors. Actions taken based on human factors analysis included the revision of ambiguous protocols; use of standardized, more self-explanatory terminology; improved access to computers and printers; and modification of the computer interface to guide users through the ordering process more smoothly. Implementation of these actions reduced heparin errors by 37.8% (Peterson et al., 2008).
Summary
While newer parenteral agents now provide preferred alternatives for anticoagulation, heparin will likely continue to play an important role in clinical situations where a shorter-acting, reversible agent is needed, e.g., when bleeding risks are high or invasive procedures require rapid adjustments in anticoagulation intensity. Implementation of safety recommendations and other measures can help to improve safety and heparin therapy, which can be expected to contribute to improved outcomes.
"Improving Heparin Safety," a recorded webcast by W. E. Dager, R. C. Gosselin, R. Raschke, and T. Vanderveen, available at www.cardinalhealth.com/clinicalcenter — click on webcasts.
"Implementing NPSG 3E," a guide prepared by members of the Clinical Affairs and the Quality and Regulatory Affairs teams of Cardinal Health, available at www.cardinalhealth.com/clinicalcenter — click on NPSG 3E Resources.
|
|
William Dager received his doctorate in pharmacy from the University of California, San Francisco (UCSF), and served his residency at the University of California, Davis Medical Center (UCDMC) in Sacramento. He also completed a Nephrology Pharmaceutical Care Preceptorship at the University of Pittsburgh, School of Pharmacy. Dager currently holds two academic positions, one as clinical professor of pharmacy at UCSF School of Pharmacy and the other as a clinical professor of Medicine at the UCD School of Medicine. As a clinical specialist at UCDMC he is responsible for difficult cases in anticoagulation, pharmacokinetics, or other critical care related situations. He also is clinically active with the cardiology service.
Dager is a fellow of the California Society of Hospital Pharmacists. He also currently serves as an instructor and regional affiliate faculty in ACLS for the American Heart Association and as chair of the Editorial Advisory Board panel on anticoagulation for the Annals of Pharmacotherapy. He is also a site coordinator for the ASHP foundation anticoagulation preceptorship.
Robert Gosselin is a technical coagulation specialist at the University of California, Davis Health System. He started working in the coagulation laboratory in 1977, while assigned to the U.S. Naval Hospital in San Diego. After his naval discharge, he passed the California boards to become a licensed medical technologist. He has worked in the U.C. Davis Health System department of pathology since 1988. Since that time, he has been involved in numerous research studies, focusing mainly on technical coagulation issues and the impact of trauma and disease on coagulation. In 1995, he received board certification from the International Board of Clinical and Applied Hemostasis, Thrombosis and Vascular Medicine. He has authored or co-authored more than 50 peer-reviewed publications, 8 distant learning courses, and currently is an active member of the International Society of Thrombosis and Hemostasis.
Robert Raschke is director of critical care services at Banner Health in Phoenix, Arizona. He holds a faculty appointment at the University of Arizona as assistant professor of clinical medicine. He received his master's degree from the University of Michigan in clinical research design and medical biostatistics and is board-certified in Internal Medicine and Critical Care Medicine.
Tim Vanderveen is vice president of the Center for Safety and Clinical Excellence. He is responsible for ensuring Cardinal Health's commitment to education and innovation to reduce variation in clinical practice, and to supporting hospitals' patient safety initiatives. Prior to this position, Vanderveen was the director of clinical affairs, medication management systems, for ALARIS Medical Systems. He has been instrumental in the development of many of the innovations and safety and performance enhancements in drug infusion.
Vanderveen served a hospital pharmacy residency at Bronson Methodist Hospital in Kalamazoo, Michigan. From 1972 to1983 he was on the faculty of the College of Pharmacy at Medical University of South Carolina and was Director of the Division of Clinical Pharmacy. He also had a faculty appointment in the College of Medicine and was on staff at the Charleston VA Hospital.Vanderveen received his BS and MS degrees from Purdue University School of Pharmacy and his PharmD degree from the Medical University of South Carolina. He may be contacted at tim.vanderveen@cardinal.com.
References
Anand, S., Ginsberg, J. S., Kearon, C., et al. (1996). The relation between the activated partial thromboplastin time response and recurrence in patients with venous thrombosis treated with continuous intravenous heparin. Archives of Internal Medicine, Aug 12-26;156(15),1677-1681.
Anand, S. S., Bates, S., Ginsberg, J. S., et al. (1999). Recurrent venous thrombosis and heparin therapy: an evaluation of the importance of early activated partial thromboplastin times. Archives of Internal Medicine, Sep 27;159(17), 2029.
Bates, D. W., Vanderveen, T., Seger, D. L., et al. (2005). Variability in intravenous medication practices: implications for medication safety. Joint Commission Journal on Patient Safety and Quality, 31(4), 203-210.
Bond, C. A., & Raehl, C. L. (2004). Pharmacist-provided anticoagulation management in United States hospitals: death rates, length of stay, Medicare charges, bleeding complications, and transfusions. The Annals of Pharmacotherapy, 24(8), 953-963.
College of American Pathologists. (2008). CGS4-A Participant Summary, College of American Pathologists, Northfield, IL.
Cardinal Health CQI Consulting Services. (2008). Cardinal Health Hospital Pooled Data.
Dager, W. E., Dougherty, J. A., Nguyen, P. H., Militello, M. A., & Smythe, M. A. (2007). Heparin-induced thrombocytopenia: a review of treatment options and special considerations. Pharmacotherapy, 27, 564-587.
Dalen, J. E., & Hirsh, J. (Eds.). (1995). Fourth ACCP Consensus Conference on Antithrombotic Therapy. Chest, Oct 108 Suppl 225-522.
Favaloro, E. J., Bonar, R., Sioufi, J., et al. (2005). Royal College Pathologists of Australasia Quality Assurance Program in Haematology. An international survey of current practice in the laboratory assessment of anticoagulant therapy with heparin. Pathology, 37, 234-238.
Hull, R. D., Raskob, G. E., Brant, R. F., et al. (1997). Relation between the time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis. Archives of Internal Medicine, Dec 8-22, 157(22), 2562-2568.
Janatpour, K. A., Gosselin, R. C., Dager, W. E., et al. (2007). Usefulness of optical density values from heparin- platelet factor 4 antibody testing and probability scoring models to diagnose heparin-induced thrombocytopenia. American Journal of Clinical Pathology, 127, 429-433.
Owings, J., Bagley, M., Gosselin, R., et al. (1996). Effect of critical injury on plasma antithrombin activity: Low antithrombin levels are associated with thromboembolic complications. The Journal of Trauma, 41(3), 396-405.
Peterson, C., Ham, C. W., & Vanderveen, T. (2008). Improving heparin safety: A multidisciplinary invited conference. Hospital Pharmacy, 43(6), 491-497.
Raschke, R. A., Reilly, B. M., Guidry, J. R., et al. (1993). The weight-based dosing nomogram compared with a "standard care" nomogram. A randomized controlled trial. Annals of Internal Medicine, 119, 874-881.
Raschke, R. A., Gollihare, B., & Peirce, J. C. (1996). The effectiveness of implementing the weight-based heparin nomogram as a practice guideline. Archives of Internal Medicine, 156, 1645-1649.
Raschke, R. A., Hirsh, J., & Guidry, J. R. (2003). Suboptimal monitoring and dosing of unfractionated heparin in comparative studies with low-molecular-weight heparin. Annals of Internal Medcine, 138, 720-724.
Santell, J. (2008). USP Medmarx data 1/1/2003-12/31/2007."Improving Heparin Safety," San Diego, CA, March 13.
The Joint Commission (2008). National Patient Safety Goals, www.jointcommission.org/GeneralPublic/NPSG/08_npsgs.htm
Wheeler, A., Jaquiss, R. D. B., & Newman, J. H. (1998). Physician practices in the treatment of pulmonary embolism and deep venous thrombosis. Archives of Internal Medicine, 148, 1321-1325.
Williams, C. K., Maddox, R. R., Heape, E., et al. (2006). Application of the IV Medication Harm Index to assess the nature of harm averted by ''smart'' infusion safety systems. Journal of Patient Safety, 2, 132‚139.
|
 |
 |
 |


















|
 |



