 |
 |
 |

January / February 2005

Technology & Quality
Usable Evidence-Based Guidelines...
for Real

Barry P. Chaiken, MD, MPH, FHIMSS
For more than two decades, clinicians struggled to use evidence-based guidelines. Ever since Wennberg's work back in the 1970s demonstrating the huge practice variation seen even in relatively small geographic areas, quality experts have worked to promote guideline use among physicians to ensure that interventions on patients actually did them some good.
Throughout the 1980s, managed care organizations did everything possible to get physicians to use guidelines in an effort to enhance quality and control costs. Their efforts included education, guidelines distribution, economic incentives, and patient education.
Unfortunately, the evidence on guideline use shows poor acceptance. In addition, numerous experts state that it takes up to 17 years for a new, effective treatment procedure to be widely adopted. This may be an exaggeration, but surely it takes longer than required. In the meantime, patients receive inappropriate, unhelpful, or even harmful care while valuable resources are wasted.
Difficult to Remain Up-to-Date
To be fair, the rapid advancement of medicine makes it nearly impossible for any physician to remain completely up-to-date on clinical progress. Also, with such rapid change, it is difficult for physicians to separate the valuable information from the less valuable or even suspect medical knowledge that deserves little attention. Lastly, the format that medical information normally comes in — journal articles and medical reports — is in the wrong information format to be integrated into a typical medical practice. For example, it requires a huge effort for a physician to evaluate a single peer-reviewed article on a specific aspect of a disease process, and then apply that single bit of knowledge to a small subset of patients within a practice panel.
All clinicians at one time or another have worked on guideline development. Their experiences have probably been similar: about 80% to 90% of the guideline is developed rather quickly, while the remaining portion is labored over for weeks, months, or even years. Often the guidelines are never completed or, if completed and disseminated, rarely used. We all know that it is easy to develop guidelines, but very hard to develop high-quality, respected, and most importantly, usable ones. In addition, upkeep of the guidelines usually never gets done.
With the advent of computerized physician order entry (CPOE) and high-capacity personal digital assistants (PDAs), there is technology that can effectively deliver guidelines (See References). A new Web technology standard, the InfoButton, offers access to context-sensitive help within clinical applications, a very effective and useful way to deliver clinical content at the point of care. The challenge exists to develop high-quality, effective, and easy-to-use guidelines that can be deployed intelligently, consistent with workflow, and at the point of care.
Clinical Evidence
Through an effort begun in 1995 by the United Kingdom's National Health Service and the British Medical Journal (BMJ) Publishing Group, there now exists a "formulary" of evidence-based medical knowledge. The current iteration of the guidelines, named Clinical Evidence (www.clinicalevidence.com), covers over 180 clinical conditions and evaluates over 2,148 treatments. Major disease categories covered are listed in Figure 1.
Clinical Evidence is available in two print versions, full and concise. The release of each version is staggered so that any changes in the guidelines can be included in the next version. It is also available online via a Web-based interface (www.clinicalevidence.org), as well as through a synchronized PDA device.
Guidelines are updated continuously with full literature searches in each topic every 12 months. As new information becomes available, it is incorporated into the Web-based version of Clinical Evidence and subsequently the next print version.
Driven by Clinical Questions
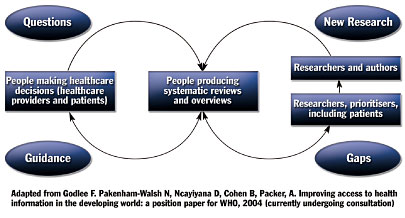
Unlike most guidelines, which are driven by clinical research, Clinical Evidence is driven by clinical questions. The literature review and other research done by the guideline developers works to answer clinical questions through presentation of medical knowledge in a format that is easy to understand, access, and use. As can be seen in Figure 2, clinical questions identify gaps in knowledge that lead to research, dissemination of knowledge to clinicians, use of that knowledge by clinicians, and identification of additional gaps in the knowledge that lead to questions and further research.

|
 |
 |
 |



















|
 |



